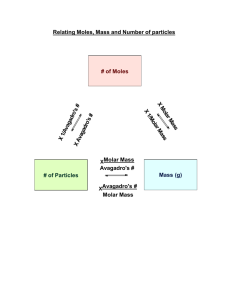
Molar Mass & Mole Conversions Practice
advertisement

Name: __________________________________ Date: ____________ Block: __________ Molar Mass & Mole Conversions Practice Milk of magnesia has several medicinal purposes. It can be used as an antacid to relieve indigestion and heartburn by neutralizing excess acid in the stomach. The chemical formula for milk of magnesia is Mg(OH)2. Complete the following questions about milk of magnesia. Show all work, use dimensional analysis when needed, and include all units and symbols throughout your work. 1. What type of compound is Mg(OH)2? a. Binary molecular c. Ionic variable b. Ionic fixed 2. What is the chemical name for Mg(OH)2? _____________________________________ 3. Calculate the molar mass of Mg(OH)2. 4. Set up an equality statement for Mg(OH)2. ______ g Mg(OH)2 = ____ moles Mg(OH)2 = _______________ formula units Mg(OH)2 5. How many grams of Mg(OH)2 are in 15.3 moles Mg(OH)2? 6. How many formula units of Mg(OH)2 are in 15.3 moles Mg(OH)2? 7. Complete the following equality: 1 formula unit Mg(OH)2 = _______ atoms H. 8. How many atoms of hydrogen are in 15.3 moles of Mg(OH)2?