Post-Lab Questions
advertisement
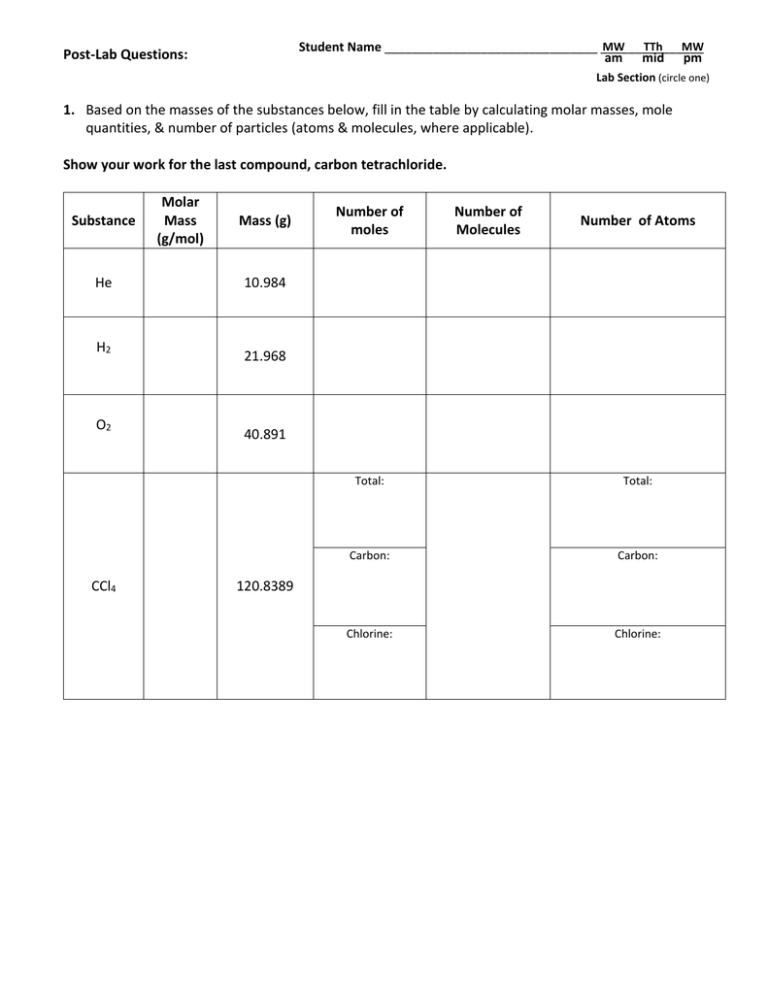
MW TTh MW Student Name _______________________________ _______________ am mid pm Post-Lab Questions: Lab Section (circle one) 1. Based on the masses of the substances below, fill in the table by calculating molar masses, mole quantities, & number of particles (atoms & molecules, where applicable). Show your work for the last compound, carbon tetrachloride. Substance He H2 O2 CCl4 Molar Mass (g/mol) Mass (g) Number of moles Number of Molecules Number of Atoms 10.984 21.968 40.891 Total: Total: Carbon: Carbon: Chlorine: Chlorine: 120.8389 2. Consider 5.443 g of the substance lead (II) nitrate. a) How many moles of Pb(NO3)2 formula units are present in this mass? How many formula units does this represent? 1 formula unit = 1 Pb(NO3)2 b) How many moles of Pb atoms are present in this mass? How many Pb atoms does this represent? c) How many moles of nitrate ions are present in this mass? How many nitrate ions does this represent? d) How many moles of oxygen atoms are present? How many O atoms does this represent? 3. A. The distance (based on a direct path) between San Francisco and Los Angeles is 558.68 km. Calculate how many iodine atoms (diameter = 2.66 x 10-10 m) would be needed to stack end to end to span this distance. 3. B. The density of solid iodine is 4.93 g/cm3. What volume of solid iodine would contain the same number of iodine atoms as calculated Part A above? 4. Assume you won the lottery and were award one mole of US dollars. If you spent one billion dollars per second, how many years would it take to run out of your lottery winnings? 5. The density of aluminum is 2.71 g/cm3. How many aluminum atoms are contained within 1.50 grams of aluminum? 6. Using information from the previous problem calculate the volume occupied by a single aluminum atom. 7. The average energy required to break a carbon-carbon single bond is 6.49 x 10-19 Joules. Calculate the energy required to break 1.00 moles of carbon-carbon single bonds. 8. How many TOTAL atoms are contained within a sample of water with a volume of 14.78 mL (1 tablespoon)? Assume a density of 0.9982 g/mL. Compare this to the estimated number of stars in our universe (approximately 10,000,000,000,000,000,000,000 stars)—which is larger?



