MICHR Letter of Support template detailing resources for trainees
advertisement

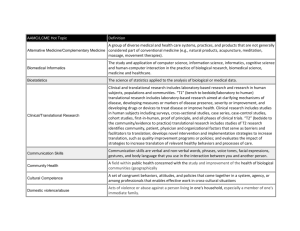
MICHR Letter of Support template detailing resources for trainees and mentors Complete template and email to MICHRLetter@umich.edu; a signed PDF on letterhead will be returned via email within 7 business days Please call the MICHR Research Navigator at 998-7474 with any questions or concerns Date [Addressee’s full campus address] Dear [preferred form of address]As Director of the Michigan Institute for Clinical & Health Research (MICHR) and Associate Dean for Clinical and Translational Research in the Medical School, it gives me great pleasure to write this letter of support for [project title]. This project… [brief project description]. I believe MICHR is an important component of the excellent research environment here at the University of Michigan (U-M), which will foster the success of your endeavors. In addition to services such as regulatory support, biostatistical analysis, outpatient and mobile nursing services, specimen storage and analysis in our biorepository, and biomedical informatics, MICHR houses a robust Education and Mentoring Program and Pilot Grant Program. This provides myriad resources for trainees, early career faculty, and mentors. For faculty, fellows, students, and staff conducting research, MICHR offers a broad array of workshops and webinars focusing on career development topics, team science, regulatory issues, responsible conduct of research, grant writing and the grant review process, and more. For example, Navigating the Regulatory Environment at U-M is a short series of online video modules introducing early- and mid-career faculty new to U-M to crucial partners in the research process. To meet the responsible conduct of research requirement established by the National Institutes of Health, MICHR has also created a workshop series, RCR4K, that provides 8 hours of face-to-face, project-based RCR education. The Research Basics for Study Coordinators workshop series introduces new research staff to the fundamentals of study development and clinical research language and culture, good clinical practice, recruitment and retention, and informed consent. For faculty and staff interested in intensive research training programs, two options are available. First, MICHR offers the Practice-Oriented Research Training (PORT) program, a combination of didactic and experiential education on research methods. Using a team-based learning approach, the PORT program teaches the fundamental skills of research design, grant preparation, research conduct, and dissemination of results. Second, in partnership with the Program in Biomedical Sciences, MICHR supports the Translational Research Education Certificate (TREC) Program, a 12 credit hour graduate certificate designed for basic scientists that provides an overview of concepts in translational science. Faculty and staff may request permission to enroll in or audit any of the courses, particularly in the gateway course and journal club course. Students may be interested in MICHR’s Summer Immersion Program in Clinical Research and Health Disparities, a 10-week hands-on learning experience. In addition, students in professional degree programs may also explore the Master of Science in Clinical Research, a one-year program housed in the School of Public Health. For postdoctoral investigators, our Postdoctoral Translational Scholars Program (PTSP) is a competitive, multidisciplinary career development award designed to prepare individuals with a PhD in a biomedical science or social science discipline for independent careers in translational research. The PTSP accomplishes this by selecting 10 scholars each year to conduct a two-year immersion in a clinical research setting, preparing them to bridge the worlds of basic and clinical science. PSTP could provide an ideal fit for PhD-trained candidates seeking to contribute to translational research. For early career faculty, our Mentored Clinical Scientists Career Development (MICHR K) award is a competitive two-year award to provide protected time for a clinician scientist to focus on conducting clinical research and is supported by a combination of funds from MICHR and the candidate's base department. This program is an excellent vehicle for junior faculty and promising emerging trainees to solidify their knowledge base and track record for a productive academic career in clinical and translational research. In support of mentoring, MICHR offers a variety of resources, including a mentor-mentee agreement intended to provide a framework for career development, talks and workshops focusing on mentoring, and an annual Distinguished Mentor Award. Mentoring is an integral component of all MICHR programs. Finally, MICHR houses a robust Pilot Grant Program, which can be a crucial source of research support and career advancement for trainees. The program has awarded more than $17 million since its inception in 2006, in conjunction with more than 100 partnering departments, colleges, schools, and centers. Approximately 30 grants are funded each year, ranging from $5,000 to $75,000 per award. The program has been very successful, issuing more than 400 awards and leading to more than $114 million in external funding. The program is aimed particularly at junior faculty and includes a detailed and supportive review process. To further help faculty succeed in building their research careers, MICHR offers a Research Development Core (RDC), providing free consultations on study design, biostatistics, and research directions. RDC also offers grant editing assistance. I believe our rich educational offerings are a demonstration of the overall commitment of U-M to build a thriving clinical and translational research enterprise. MICHR is a crucial component of the infrastructure and environment in which proposals such as yours can succeed. Congratulations on your exciting proposal, and I wish you much success with the application. All of us here at MICHR look forward to working with you in the coming years. Sincerely, Thomas P. Shanley, M.D. Ferrantino Professor of Pediatrics and Communicable Diseases Director, Michigan Institute for Clinical and Health Research Associate Dean for Clinical and Translational Research, Medical School