Chemical Equations
advertisement

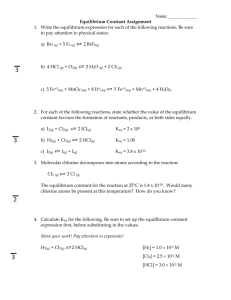
Final Exam Review Although this exam is only over second semester, there are concepts from first semester that are the basis of concepts for second semester. Some of these are: formula writing, density, ionic and covalent compounds. (Also, if I have forgotten a topic I reserve the right to update this list) Chemical Equations Name chemicals when given the formula and write correct formulas for chemicals when given their names (covalent compounds, ionic compounds, acids, bases, etc.) Know which elements are diatomic Know the state of matter for all elements Know the common indications or evidence of a chemical reaction Write chemical equations when given the reaction in words Know the meaning of different symbols used in a chemical equation (+, , , Δ, s, l, g, aq, etc) Balance chemical equations Know and be able to identify common characteristics of each of the 6 types of reactions discussed in class Classify chemical equations as 1 of the 6 types discussed in class Predict the products of a chemical reaction when given the reactants Use the Activity Series to determine whether a single replacement reaction will occur Use the Solubility Table to determine if an ionic compound is soluble (aq) or insoluble (s) Write complete and net ionic equations for single and double replacement reactions Stoichiometry Understand how to calculate: moles, molar mass, how to calculate moles of using the liters of a gas using the conversion 22.4L=1mole Use mole ratios to calculate moles, grams of reactants and products in a chemical reaction Limiting reagent Empirical formul Molecular formula Solutions: basic terminology – solute, solvent, dilute, concentrated, saturated, unsaturated 8 methods used in class to measure the concentration of a solution – know how to calculate any of the variables in each of these : molarity (M) = molality (m) = normality (N) = parts per million (ppm) = or parts per billion (ppb) mass percent (m/m) = mass/volume percent (m/v) = volume percent (v/v) = mole fraction = how proof relates to % v/v in an alcoholic beverage where and when certain units of concentration are most frequently used how to create a solution with a specific molarity in the lab use M1V1 = M2V2 to solve problems and to perform a dilution in the lab understand what it means to “dilute” a solution EQUILIBRIUM AND REACTION RATES REVIEW EQUILIBRIUM - Differentiate between an irreversible rxn () and a reversible rxn (↔) What is chemical equilibrium? o What are the conditions necessary for it to be achieved? o How do the two reaction rates compare at equilibrium? o What chemicals are present in the reaction? (reactants, products, both?) o What is the difference between static and dynamic equilibrium? o How does the Keq of a forward rxn mathematically relate to Keq of the reverse rxn? - Correctly write chemical reactions, Keq, expressions - Calculate Keq for a rxn when given concentrations - Use a given Keq value to find the concentration of a substance at equilibrium - What does the size of Keq mean? What if Keq >, <, or = 1? - Explain how the concentrations of chemicals change and when they remain constant from the beginning of a rxn until it reaches equilibrium - Explain and apply Le Chatelier’s Principle to a system in equilibrium with respect to various stresses - Predict equilibrium shifts, changes in reactant/product concentrations, and changes to Keq based on Le Chatelier’s Principle when stresses are applied - What is ∆H? What does it mean when it is + or -? Endothermic or exothermic? Is heat considered to be a reactant or product? REACTION RATES - - What are chemical kinetics? What is the rate of a chemical rxn? How is it found? Use graphs, data, stoichiometry and/or balanced chemical equations to calculate rxn rates of individual reactants/products Explain how and why temperature, concentration, pressure (for gas), and surface area affect rxn rates – don’t just simply know that these are the main factors affecting rates Know other factors that can affect the rate of a rxn, including catalysts, inhibitors, orientation of molecules, stirring, etc. What is activation energy? What things must occur for a rxn to actually happen? Interpret an activation energy diagram with respect to Ea, ΔH, a catalyst, PE, exothermic vs. endothermic, starting energy, etc. What does a catalyst do? What is an enzyme? Where are they ACIDS/BASES Know general properties/characteristics of acids and base Explain strong vs. weak acids and bases Know the difference between strong and concentrated acids/bases Explain the self-ionization of water – be able to write the equation(s) to show it Use Kw to calculate [H+] or [OH-] Know the pH, [H+] and [OH-] of a neutral solution What does pH stand for? What about pOH? Explain the pH/pOH scale and use them to classify a substance as acid, base, or neutral Know how the [H+] and [OH-] compare in acidic, basic, and neutral solutions Can pH and pOH ever be <0 or >14? Calculate pH, pOH, [H+], [OH-] What is an indicator?