EZRA_Letterhead_TS
advertisement

March 4, 2013
Norman Stockbridge, M.D. Ph.D.
Director
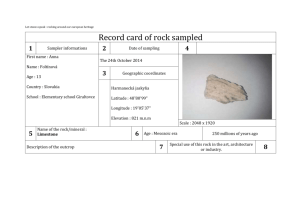
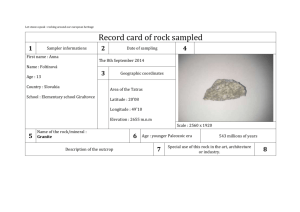
Office of Drug Evaluation I
Division of Cardiovascular and Renal Products
10903 New Hampshire Avenue
Silver Spring, MD 20993
Subject: Request for Type B PRE-IND Meeting – Valsartan ER Tablets
Dear Dr. Stockbridge:
Section 119(a) of the FDA Modernization Act of 1997 (FDAMA) directs the Food and Drug
Administration (FDA) to meet with the sponsor of an investigation or the applicant for a
drug if the sponsor or applicant makes a “reasonable written request for a meeting for the
purpose of reaching agreement on the design and size of clinical trials intended to form the
primary basis of an effectiveness claim.” Pursuant to the implementation of section 119(a),
the FDA issued a guidance document titled “Formal Meetings With Sponsors and
Applicants for PDUFA Products,” in which the agency describes the procedures a sponsor
or applicant should follow when filing a written request. With this letter, and in accordance
with both FDAMA section 119(a) and FDA’s Guidance document, EZRA Pharma dba,
Division of EZRA Innovations LLC (“EZRA”) requests a Type B PRE-IND meeting with the
FDA for its product Valsartan ER Tablets for the treatment of Stage I and Stage II
hypertension (HTN).
1.0 Product Name
Valsartan Extended Release Tablets 160 mg and 80 mg
Interchangeably referenced as Valsartan ER and Valsartan XL Tablets.
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
2.0 Chemical Names and Structure
Valsartan USP
Molecular Formula: C24H29N5O3
Molecular Weight: 435.51876
(S)-N-valeryl-N-{2’-(1H-Tetrazol–5–yl) biphenyl-4-yl] methyl}valine
OR
N-[p-(o-1H-Tetrazol-5-ylphenyl)benzyl]-N-valeryl-L-valine (Valsartan USP)
IUPAC Name: (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl)phenyl]phenyl]
methyl]amino]butanoic acid
CAS: 0137862-53-4
3.0 Proposed Indication
Treatment of Hypertension (HTN)
4.0 Type of Meeting Requested
Type B pre-IND You use “pre” in all caps in other places in the document. Should these
be caps or no?
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
5.0 Statement Purpose of Meeting
1. Confirm that 505(b)(2) NDA is the appropriate filing mechanism.
2. Discuss the sponsor’s plans to demonstrate safety and efficacy via existing
pharmacokinetics (PK) studies and literature.
3. Confirm that the existing scientific literature is sufficient such that no additional
clinical safety or clinical safety/efficacy studies are required.
4. Review the requirements for the IND filing.
5. Discuss the Chemistry Manufacturing and Control (CMC) and Phase 1 clinical
protocol synopsis questions presented in this Meeting Package and to
address any unresolved issues related in the proposed IND.
EZRA is presenting a brief overview of the dosage form under development with a
summary of six pharmacokinetics (PK) studies conducted on the product. EZRA is
also providing a brief synopsis of the proposed Phase 1 clinical study as supporting
information to facilitate the meeting request. Supporting information on CMC and
Phase I clinical design is provided in the attachment 1 of this request letter.
6.0 List of Specific Outcomes Expected From the Meeting
Agreement that 505(b)(2) NDA is the appropriate filing mechanism for
Valsartan ER Tablets.
Agreement that the sponsor’s plan to demonstrate safety and efficacy via
existing PK studies and literature is sufficient.
Confirm that the existing scientific literature is sufficient such that no
additional clinical safety or clinical safety/efficacy studies are required.
Review the requirements for the IND filing.
Discuss the Chemistry Manufacturing and Control (CMC) and Phase 1
clinical protocol synopsis questions presented in this Meeting Package and
to address any unresolved issues related in the proposed IND.
7.0 Proposed Agenda Items
1.
2.
3.
4.
5.
Brief introductions (All)
Overview of the development of the product and rationale
Presentation of the Phase 1 study design
Open discussion of Agency responses to list of questions
Unresolved issues
5 minutes
10 minutes
20 minutes
40 minutes
15 minutes
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
8.0 Draft List of Questions
8.1 General/Regulatory
a. Does the Agency agree that a 505(b)(2) is the appropriate filing mechanism
for Valsartan ER Tablets?
b. Does the Agency agree that Diovan® Tablets 160 mg of Novartis
Pharmaceuticals Corporation (“Novartis”) is an appropriate reference listed
drug (RLD) strength to use for Phase I clinical and subsequent Phase 3
clinical studies.
c. Does the Agency agree that no additional pre-clinical safety studies will be
required?
8.2 Non-clinical
The sponsor proposes to support the safety of EZRA’s Valsartan ER Tablets
in the following manner:
a. The approval of an NDA for Diovan® brand of Valsartan Tablets 160 mg
(NDA 021283) approved on July 18, 2001 owned by Novartis
Pharmaceuticals Corporation.
b. Available literature on safety and efficacy of Valsartan Tablets.
8.3 Clinical
EZRA proposes to evaluate the superior therapeutic efficacy and safety of Valsartan
ER Tablets for the treatment of HTN.
Phase I: A prospective, randomized, open label, blinded endpoint, crossover study
to compare the safety, efficacy, and duration of action of Valsartan ER 160 mg
once-daily to Diovan® 160 mg once-daily on patients with Stage I and Stage II
hypertension in approximately 30 patients.
Phase 3: Clinical trial design to be determined after the outcome of Phase 1 study.
a. Does FDA agree with the endpoints identified in Phase 1 Protocol Synopsys
that prove the product effective?
b. Does FDA agree with the use of the initial Ambulatory Blood Pressure
Monitoring (ABPM) baseline as the baseline for the study?
c. Does Agency agree with the dosing strategy of Diovan® Tablets without food
and Valsartan ER Tablets with food, that is the dosing of each agent relative
to meals, based on PK summary data provided?
d. Does Agency agree with the selection of 160 mg as the principal dose?
e. Does Agency agree with the three weeks long for each treatment?
f. Does Agency agree with the 1- week washout period between treatments?
g. Does Agency agree with conducting Phase 3 clinical trial after successful
outcome of Phase 1 study and would it to be sufficient for the approval of
505(b)(2) NDA?
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
8.4 Chemistry, Manufacturing and Controls (CMC)
EZRA proposes to manufacture a 10,000 tablets batch size under cGMP for Phase I
clinical study. The formulation and process would essentially be same as the future
larger scale batch that will be used for Phase 3 clinical trials.
EZRA proposes to submit minimum of two months’ accelerated stability data for
non-GMP laboratory scale batch as per ICH Guidance with the IND while continuing
to generate stability data on the cGMP batch for Phase 1 clinical study. Before the
start of Phase 1 study EZRA will have minimum of 1 month accelerated stability
data on this cGMP batch. EZRA will provide assurance for the acceptable batch
stability during the entire time interval of the Phase I study.
a. Does Agency agree with the use of the clinical supply for Phase 1 study of a
10,000-tablet batch size?
b. Does Agency agree with the stability data submission strategy provided?
9.0 Meeting Attendees
9.1 EZRA Attendees
Michel Geranen
Chief Executive Officer
Shirish A. Shah, PhD, RAC
Chief Scientific Officer – Development and
Regulatory
Joseph A. Fix, MBA, PhD
Chief Operating Officer
9.2 Consultant Attendee
Joel M. Neutel, MD
Clinical Professor of Medicine
University of California at Irvine
Director of Research
Orange County Research Center
9.3 Requested Agency Attendees
Norman Stockbridge, MD, PhD
Director, Div. of Cardiovascular and Renal
Products (DCRP)
Edward Fromm
Quynh Nguyen, MD
Supervisory Project Manager
Director of Regulatory
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
Note: Project Manager, Medical Reviewer, CMC Reviewer are to be
designated by the DCRP.
10.0 Suggested Meeting Dates and Times
EZRA proposes the following dates and times:
April 16 – 18, 2013 in the afternoon
April 23 – 25, 2013 in the afternoon
11.0 Approximate Date of Submission of Briefing Document
The briefing document will be submitted to the Agency four weeks prior to the date
of the pre-IND meeting.
If you have any questions or need further information, please contact me at 480-659-8963.
Sincerely,
Shirish A. Shah, PhD; RAC
Chief Scientific Officer
Development and Regulatory
EZRA Pharma dba
Div. of EZRA Innovations LLC
Phone: +1 480 659 8963
Fax: +1 855 424 9113
Mobile: +1 480 245 8311
sshah@ezrapharma.com
Mailing Address:
2814 W Wildwood Drive
Phoenix, AZ 85045
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205
Corporate: EZRA Pharma DBA 4301 W. Markham Street #831 Little Rock, AR, 72205
Courier:
401 South Cedar Street Little Rock, AR, 72205