Entresto (Sacubitril/Valsartan) Presentation: Heart Failure Treatment
advertisement

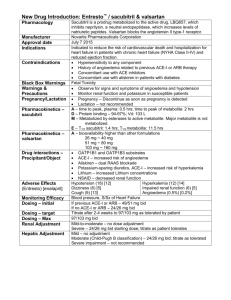
® Entresto (sacubitril & valsartan) Manufacturer: Novartis Pharmaceuticals Corporation FDA Approval Date: July 7 2015 Entresto® - sacubitril/valsartan Clinical Application • Indications: • Neprilysin inhibitor and angiotensin II receptor blocker combination to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction • Place in therapy: • Patient who have progressed in severity of their heart failure on optimum ACE inhibitor therapy Entresto® - sacubitril/valsartan Clinical Application • Contraindications • Hypersensitivity to any component • History of angioedema related to previous ACE inhibitor or ARB therapy • Concomitant use with ACE inhibitors • Concomitant use with aliskiren in patients with diabetes Entresto® - sacubitril/valsartan Clinical Application • Warnings & precautions • Observe for signs and symptoms of angioedema and hypotension • Monitor renal function and potassium in susceptible patients Entresto® - sacubitril/valsartan Clinical Application • Pregnancy – contraindicated • Lactation – not recommended Entresto® - sacubitril/valsartan Drug Facts • Pharmacology: • Sacubitril – prodrug metabolized to active metabolite (LBQ657), which inhibits neprilysin • Neprilisyn – neutral endopeptidase • Leads to increase in level of peptides, including natriuretic peptides • Valsartan – blocks the angiotensin II type-1 (AT1) receptor Entresto® - sacubitril/valsartan Drug Facts • Pharmacokinetics: A D M E Time to peak: 0.5 hrs Time to peak of metabolite: 2 hrs Protein binding – 94-97% Vd: 103 L Metabolized by esterases to active metabolite Major metabolite is not metabolized T1/2 sacubitril: 1.4 hrs T1/2 metabolite: 11.5 hrs Entresto® - sacubitril/valsartan Drug Interactions Precipitant Entresto* • Drug Object Nature of interaction Decrease AUC and Cmax Furosemide, Interactions – Object Drugs: levonorgestrel, • Object drugs are affected by “the HCTZ, metformin drug” Entresto*reviewed Atorvastatin Increase AUC and Cmax • List ( ##%) if available Entresto ACE-I Increased risk of angioedema • Ex: ASA (100%) Entresto Aliskiren Dual RAAS blockade Entresto Potassium-sparing Increased risk of diuretics, ACE-I hyperkalemia NSAID Entresto Decreased renal function Entresto Lithium Increased concentrations Entresto® - sacubitril/valsartan Adverse Effects Side effect Angioedema Hypotension Impaired renal function Hyperkalemia Cough Entresto 0.5% 18% 6% Enalapril 0.2% 12% 5% 12% 9% 14% 13% Entresto® - sacubitril/valsartan Monitoring Parameters • Efficacy Monitoring: • Blood pressure at each visit and dose titration • Toxicity Monitoring: • Serum electrolytes (K+) • SCr Entresto® - sacubitril/valsartan Prescription Information • Dosing: initial • Previous ACE-I or ARB – 49/51 mg bid • No ACE-I or ARB or low doses – 24/26 mg bid • Dosing: target • Titrate after 2-4 weeks to 97/103 mg bid as tolerated by the patient Entresto® - sacubitril/valsartan Prescription Information • Renal impairment • Mild-moderate – no dose adjustment • Severe – 24/26 mg bid (initial) • Hepatic impairment • Mild – no adjustment • Moderate (Child-Pugh B) – 24/26 mg bid (initial) • Severe impairment – not recommended Entresto® - sacubitril/valsartan Prescription Information • If switching from ACE-I to Entresto, 36 hour washout period is recommended • Cost – Source: NY Times; Accessed 8/21/15 • $4,500/year • Novartis offers free 30-day supply and $10 co-pay cards Entresto® - sacubitril/valsartan Literature Review PARADIGM-HF • Purpose: To compare the combination of sacubitril/valsartan with enalapril in patients who have HFrEF • Design: randomized, double-blind, phase 3 trial • 1043 sites in 47 countries McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review Inclusion Criteria • Inclusion Criteria Exclusion Criteria • Age >18 years • Symptomatic hypotension •• NYHA Exclusion class II-IVCriteria • SBP <100 mg • Ejection fraction <40% (amended to <35%) • eGFR <30 ml/min/1.73 m2 or eGFR >25% • BNP >150 pg/mL or proBNP >600 pg/mL • Serum K+ >5.2 mEq/L • Treatment with ACE-I or ARB • Hx of angioedema or unacceptable side effects during receipt of ACE-I or ARB McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review • Intervention: LCZ696 200 mg bid vs. enalapril 10 mg bid • Primary endpoint: composite of death from cardiovascular causes or a first hospitalization for HF • Secondary endpoint: • Time to death from any cause • Change from baseline to 8 months in clinical summary score (KCCQ) • Time to new onset atrial fibrillation • Time to first occurrence of a decline in renal function McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review • Baseline characteristics Entresto (N=4187) Enalapril (N=4212) 63.8 63.8 Female 879 (21.0%) 953 (22.6%) White 2763 (66.0%) 2781 (66.0%) Medical History: • HTN • Afib • Hospitalization for HF • MI • Pretrial use of ACE-I • Pretrial use of ARB 2969 (70.9%) 1517 (36.2%) 2607 (62.3%) 1818 (43.4%) 3266 (78.0%) 929 (22.2%) 2971 (70.5) 1574 (37.4%) 2667 (63.3%) 1816 (43.1%) 3266 (77.5%) 963 (22.9%) Treatment at randomization: • Diuretic • Beta-blocker • Mineralocorticoid antagonist 3363 (80.3%) 3899 (93.1%) 2271 (54.2%) 3375 (80.1%) 3912 (92.9%) 2400 (57.0%) NYHA class II 2998 (71.6%) 2921 (69.3%) Age McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review • Results Entresto (N=4187) Enalapril (N=4212) HR or Difference (95% CI) P-value Composite outcome 914 (21.8) 1117 (26.5) 0.80 (0.73-0.87) <0.001 Death from cardiovascular causes 558 (13.3) 693 (16.5) 0.80 (0.71-0.89) <0.001 1st hospitalization for worsening HF 537 (12.8) 658 (15.6) 0.79 (0.71-0.89) <0.001 Death from any cause 711 (17.0) 835 (19.8) 0.84 (0.76-0.93) <0.001 Change in KCCQ clinical summary score at 8 mo -2.99 -4.63 1.64 (0.63-2.65) 0.001 New-onset afib 84 (3.1) 83 (3.1) 0.97 (0.72-1.31) 0.83 Decline in renal fxn 94 (2.2) 108 (2.6) 0.86 (0.65-1.13) 0.28 McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review • Safety endpoints Entresto (N=4187) Enalapril (N=4212) P-value Symptomatic 588 (14.0) 388 (9.2) <0.001 Symptomatic w/ SBP <90 mmHg 112 (2.7) 59 (1.4) <0.001 SCr > 2.5 mg/dl 139 (3.3) 188 (4.5) 0.007 Serum K >6.0 mmol/L 181 (4.3) 236 (5.6) 0.007 Cough 474 (11.3) 601 (14.3) <0.001 Hypotension McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto® - sacubitril/valsartan Literature Review • Conclusions • Entresto’s dual inhibition was more effective in reducing the risk of death from cardiovascular causes or hospitalization for HF than ACE inhibition with enalapril • The only significant side effect was symptomatic hypotension, though this did not increase the rate of discontinuation McMurray JJV, et al. N Engl J Med. 2014;371(11): 993-1004 Entresto™ - sacubitril/valsartan Summary • Entresto™ inhibits neprilysin and angiotensin receptors • Indicated to reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction • Initial dose is based on receipt of ACE-I or ARB therapy prior to initiation • Avoid use in combination with an ACE-I or in patients with a history of angioedema • Most common side effect is hypotension Entresto® - sacubitril/valsartan References 1. Entresto [sacubitril and valsartan] package insert. Novartis Pharmaceutical Corporation. July 2015. 2. McMurray, J, et al. PARADIGM-HF Study. New England Journal of Medicine. 2014;371;11:9931004. 3. Pollack, A. The New York Times Website. F.D.A. Approves Heart Drug Entresto Said to Cut Death Risk by 20%. http://www.nytimes.com/2015/07/08/business/inte rnational/fda-approves-heart-drug-entresto-afterpromising-trial-results.html. Published July 7, 2015. Accessed August 21, 2015.