Pencils_Diamond_Graphene
advertisement

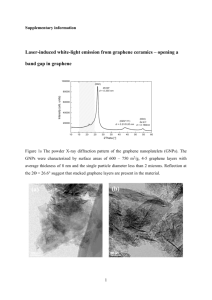
SHPE Jr. Chapter January 2015 STEM Activity Instructor resource Pencils, Diamond, Graphene Students learn about nano materials and explore the electrical properties of graphite. They learn about different allotropes of carbon and how differing arrangements of atoms in a substance can alter its electrical and physical properties. They propose uses for nanoscale graphene Learning objectives • Understand what nanotechnology is • Learn about graphene as a nanoscale material • Understand the concept of allotropes • Learn about the electrical properties of carbon allotropes • Learn about circuits and conductors • Consider how nanoscale engineering can address societal issues Engineering/STEM areas: Materials science, nanoscale science Materials • Student Resource Sheets (in lesson) • For each pair or team: • A sharpened pencil or mechanical pencil • Paper • Small LED bulb • 330 Ohm resistor • Insulated alligator clip connectors • 9-volt battery (The LED bulb, resistor, and clips can be purchased at Radio Shack) Optional: A pencil or leads from a mechanical pencil, and diamond-like jewelry (or photos of these things). Instructions for building the graphite circuit are in the Student Worksheet. Time required 45 - 60 mins Suggested group size: 2-3, depending on budget and number of students Preparation 1. Read through both the student and instructor resources so you have the background information 2. Gather all the necessary materials. Assemble sets of materials for each group 3. Make enough copies of the Student Resource so that each student has one, plus a few extras 4. Make your own setup of a simple circuit to use as a demo Procedure 1. This lesson will involve more explaining and discussion on your part, almost a mini-lecture. But first you’ll have the students do an activity to pique their interest. Begin the lesson by asking them what a diamond and a pencil have in common and how they’re different. If possible, have a picture of a pencil and a diamond, or even bring a pencil and some diamond-like jewelry. 2. Explain that both diamond and pencil “lead” are made of carbon (therefore, the “lead” isn’t actually lead. It’s a compound called graphite.), and solicit explanations of why they are so different if they are made of the same thing. 3. Explain allotropes, and cite diamond and graphite as examples. 4. Tell students that graphite and diamond differ in an invisible way that is very important to engineers. Explain that they’re going to do an activity where they demonstrate for themselves an important property of graphite. It will involved making a circuit. 5. If necessary, go over the introduction to the Student Resource (the paragraphs about circuits) and make sure students understand what a circuit is. 6. Make sure each group has a set of materials required for making the graphite circuit. Go over the first page of the Student Resource, explaining what a circuit is. Ask what students think will happen. Then circulate among student groups as they’re doing their setup. Have your circuit ready to show them as an example (but either don’t put any graphite on yours or don’t close the circuit). Make sure all students manage to get their light bulbs lit. 7. Explain that the conductivity of graphite makes it very useful, and that only a single atom layer of carbon is needed to conduct electricity. In fact, engineers have been developing new materials using a single layer of graphite, which they call graphene, Graphene is a very efficient conductor, and is one of the strongest materials known. 8. Go over the information in the Student Resource. Make sure students understand: o Nanoscale materials o The relationship between graphene and graphite o Special nanoscale properties of graphene o Potential applications that engineers can consider 9. Have each group work together to come up with applications for graphene and describe them in their student resource. Students then share their ideas with the larger group. Assessments • Have student groups devise a new application of graphene and describe it to the whole group. Their description should include: • A description of what the application looks like on a nanoscale (and a macroscale, if appropriate) • An explanation of how the special properties of graphene make this device possible • An explanation of how the special properties of graphene make this device an improvement over what already exists and/or how this device could contribute to solving a problem. Extensions • Have students research some current applications of nanoscale carbon technologies and describe the science behind how they work. • Introduce students to the types of microscopes used to visualize nanoscale materials. • Discuss the use and benefits of allotropes of elements other than carbon. For example, different allotropes of phosphorus have different industrial applications based on their conductivity and whether or not they’re flammable. • Imagine that one engineer devises a new application for carbon nanotubes, and another engineer improves upon it. Both the application and its improvement are included in a new product. Have a debate addressing how each engineer should be credited and/or compensated for their inventions. Resources/Bibliography The Power of Graphene http://tryengineering.org/lesson-plans/power-graphene Exploring Materials: Graphene http://www.nisenet.org/catalog/programs/exploring_materials__graphene_nanodays_2012 The Nanotechnology Revolution: Graphene http://www.classroomengineers.org/education/media/nanotechnology-revolutiongraphene-engineer/?ar_a=6 Carbon nanotubes find real world applications: http://phys.org/news/2014-03-carbon-nanotubes-real-world-applications.html Allotropes Explained: http://www.chemistryexplained.com/A-Ar/Allotropes.html Jan. 2015 SHPE Jr. Chapter STEM Activity Student Resource and Worksheet Pencils, Diamonds, Graphene You’re going to start this lesson by doing an activity and explaining what you see. Activity Procedure This activity has four steps: 1) Put together a simple circuit 2) Test your circuit 3) Add graphite to your circuit 4) Test the new circuit Your instructor should give you the following materials: A sharpened pencil or mechanical pencil Paper T h e P o w e r oSmall f GLED rap hene bulb 330 Ohm resistor tudent Resource: 4 Insulated alligator clip connectors What is a Simple Circuit? 9-volt battery Simple Circuit simple circuit consists of three minimum elements that are required to complete a To build the simple circuit: nctioning electric circuit: a source of electricity (battery), a path or conductor on which A circuit is a closed loop that electrons candevice travel that through. A simple circuit ectricity flows (wire) and an electrical resistor (lamp) which is any requires three elements: anaenergy sourcecontaining, (often a battery), a path for electrons ectricity to operate.requires The illustration below shows simple circuit one attery, two wires, and a bulb. The flow of electricity is from the high potential (+) to flow along (a conductive material, sometimes a wire), and a device that rminal of the battery throughelectricity the bulb (lighting it up), and back to the negative (-) requires to operate. rminal, in a continual flow. Schematic Diagram of a Simple Circuit In order for a circuit to operate, it has to be a closed, meaning that there has to be a continual path for the electrons to follow. In the illustration here, electrons flow from the battery through the wire to the bulb. As electrons pass through the bulb, they light it up. Electrons leave the bulb through another wire that takes them to the battery. If the wire was disconnected from either end of the battery, the electrons wouldn’t be able to flow through the whole loop, and the circuit wouldn’t light the bulb. he following is a schematic diagram of the simple circuit showing the electronic symbols r the battery, switch, and bulb. First, put together a simple circuit by making the following connections between the items you have: 1) Connect on end of a pair of alligator clips to cathode (positive node) of the battery. Connect the other end of that same alligator clip to one wire of the resistor. 2) Using second alligator clip, connect the other wire from the resistor to one wire on the LED bulb. 3) Using a third alligator clip, connect the other wire on the LED bulb to the anode (negative node) of the battery. Your LED bulb should light up! If it doesn’t, ask for some help with your circuit. Now, you’ll add something to your circuit and see what happens. 1) Use your pencil to fill in a space on the edge of the card or paper you’ve been provided with. The space should be 1 or 2 inches wide and go 1 inch in from the edge of the paper. 2) Disconnect the alligator clip from the anode of the battery (keep it connected to the LED bulb. Connect the newly freed end of that alligator clip to the paper so that the clip is solidly in the space you filled in with the pencil. 3) Connect one end of your fourth alligator clip to the anode of the battery. Clip the other end to the paper, also solidly in a space that you filled in with pencil, but at least a half-inch away from the other alligator clip. What happened when you connected the last alligator clip? What explanation can you think of to explain it? Pencils and diamonds: The same, but different When you look at a pencil “lead” and a diamond, do you see much resemblance? One is a clear, sparkling solid, hard enough to cut glass, and rare among the earth’s gems. The other is dull gray, soft enough to rub off on your fingers, and found in abundance. But when it comes right down to it, they’re made of the same stuff: carbon. The key is that the atoms that make up the two substances are arranged in different patterns. In the pencil “lead,” (which isn’t lead at all, but a substance called graphite), the carbon is arranged in 2-dimensional layers of atoms bound together in a pattern similar to chicken wire. In a diamond, the carbon atoms are arranged in a 3dimensional lattice. Different forms of a pure element are called allotropes. These differing arrangements affect the properties of the material—what it looks like, how hard it is, and so on. These differing arrangements affect the properties of the material—what it looks like, how hard it is, and so on. One invisible way in which diamonds and graphite differ is in their ability to conduct electricity. Diamonds are pretty ineffective in this regard, while graphite is a rather efficient conductor. In the 1980s, two Russian scientists isolated a single-atom layer of graphite by using simple adhesive tape to collect graphite from a pencil. They discovered that even this layer graphite just one atom thick can conduct electricity, and can do so even more efficiently than graphite. Engineers soon began researching how to make these layers of carbon “chicken wire,” called graphene, in a cost effective way, and exploring its properties. In 2010, the scientists won a Nobel prize for their discovery. Layers of graphite Single layer of graphene Graphene is considered a nanomaterial—that is, a material that is either created or manipulated on the atomic scale. Most of the materials we deal with in everyday life—cotton, wood, steel, etc.—are made and worked with on the macro scale. When engineers work with nanomaterials, they may move single atoms or small groups of atoms. “Nano” is a prefix used before measurements, like the prefixes “kilo,” “milli,” and “centi.” A nanometer is one-billionth, or 10-9 of a meter. A nanometer is smaller than any visible wavelengths of light, so researchers have to use special microscopes to visualize nanomaterials. Engineers have devised ways to make nanoscale structures out of graphene. They can roll a layer of graphene into a carbon nanotube, which is very light and strong for its size. Engineers are experimenting with ways to use these very strong structures in machines, cars, and sports equipment. Because they have a large surface area, they may make very effective water filters. Their electrical conductivity makes them potentially powerful in batteries and integrated circuits. Carbon nanotube The fact that carbon nanotubes are very light and strong means an airplane incorporating them might weigh less and require less fuel. A circuit with two electrodes and ten graphene nanoribbons connecting them. (http://www.gtresearchnews.gatech.edu) While most products that incorporate nanoscale carbon structures are still in the research stages, there’s no shortage of ideas of ways to make use of the special properties of these carbon allotropes. The field of nanotechnology will remain a promising area of engineering and research for many years. And you may find graphene or other nanoscale carbon structures in your phone, computer, or other electronics in the near future! Devise another application for graphene, while thinking about the following questions. You can write a description or make a drawing in the box on the next page. How would you describe your application? Can you discuss it on both the nanoscale and the macroscale? What properties of graphene make this device possible? What usual properties of graphene make this device an improvement over what already exists? How could this device contribute to solving a problem? Draw or describe your application in this box. You’ll share your idea with others when you’re done. Vocabulary • Allotrope – Materials made of the same element but with different bonding arrangements between atoms. Different allotropes have different physical, chemical, and electrical properties. • Circuit – A loop that electrons can travel through. • Carbon nanotube – A layer of graphene rolled into a tube. Carbon nanotubes are very strong. • Graphene – A layer of graphite-like carbon that is only one atom thick. Graphene is a very strong material and a very efficient conductor. • Graphite – A common compound that is an allotrope of carbon. Graphite contains many layers of carbon atoms connected in a chicken-wire type pattern. • Nanoscale – Working with or studying materials at the scale of 10-9 of a meter.