File
advertisement

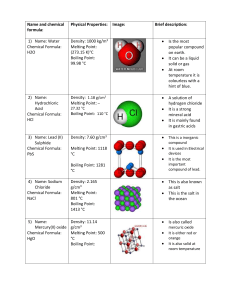
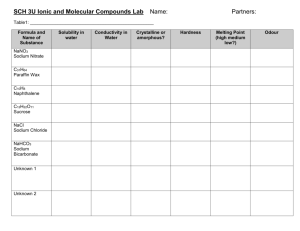
Recrystallisation of benzoic acid and determination by measuring its melting point Recrystallisation Theory An impure solid may be purified by first dissolving it in the minimum quantity of a boiling solvent. Insoluble impurities are then removed by rapid filtration of the hot mixture. The filtrate is next allowed to cool slowly. Since the solubility of the solid decreases as the temperature falls, crystals will form. The soluble impurities mostly remain dissolved in the solvent as the crystal structure “regrows” in a regular pattern. Procedure Boil about 100 cm3 deionised water in a 250 cm3 beaker and keep the water just at its boiling point. Weigh out about 1 g of the benzoic acid sample in a 100 cm3 beaker. Flute a filter paper . Place the fluted filter paper in the funnel, and secure it by wetting it. Prepare for hot filtration by standing a 250 cm3 conical flask containing about 20 cm3 of boiling water on a hotplate or over a low Bunsen flame. Sit the funnel with fluted filter paper in the neck of the flask. Using a dropping pipette, add 25 cm3 of boiling water to the beaker containing the sample, with constant stirring. Heat the mixture gently to keep the solution near its boiling point. Any remaining crystals should be dissolved by adding the minimum extra amount necessary of boiling water. When ready to filter, empty the conical flask, replace the funnel quickly, and stand the flask on a heat resistant mat. Working quickly but carefully, pour the boiling solution through the filter paper (Fig.1) in small portions. (It may be necessary to reheat the beaker periodically.) If any crystals form in the filter paper, add a little boiling water to dissolve them. Empty the filtrate into a warm 100 cm3 beaker. Heat the filtrate until traces of crystals begin to appear on the sides of the beaker. You now have the minimum amount of solvent required. Place the beaker on a heat resistant mat and allow the filtrate to cool to room temperature. To recover the crystals, carefully filter the recrystallised mixture, by vacuum filtration if possible. If some crystals remain in the beaker, transfer the filtered liquid back to the beaker and filter again. Repeat this procedure until all the crystals have been transferred to the funnel. Wash with 2 cm3 of ice cold deionised water and dry the crystals using vacuum filtration for ten minutes. Using the spatula, remove the crystals from the funnel and transfer them to a previously weighed dry clock glass. Dry the crystals by placing the clock glass in an oven set at 80 0C, or on top of a radiator for 24 hours. Melting point determination Theory One of the physical properties of a pure substance is its melting point, or, more correctly, its melting point range. The melting point range of a substance is the narrow band of temperatures between the temperature at which melting begins and the temperature at which all the solid has liquefied. The solid state and the liquid state of the substance co-exist across this small temperature range. The melting point range of an impure substance is lower and broader than that recorded for a pure sample of the same substance. Comparing the melting point ranges of the benzoic acid sample before and after recrystallisation with the melting point range of pure (Analar) benzoic acid will therefore indicate the success of the recrystallization process in improving the sample purity. It will also indicate whether it is as pure as an Analar sample. Calculate the percentage yield that you recovered from your sample. Melting point determination 1. Grind up the benzoic acid into a fine powder using the pestle and mortar. 2. Prepare a number of capillary tubes by gently breaking and sealing on end by heating in a hot Bunsen flame for a few seconds. 3. Switch on the melting point equipment . 4. Read the thermometer 5. Dip the capillary tube in the powder and place the capillary tube in the hole next to the thermometer. 6. Observe the melting point of the benzoic acid. When the compound begins to melt record its temperature. When it is completed melted record the temperature. This will give you the melting range. 7. Repeat for the re-crystallised benzoic acid. 8. Compare your results. Results Melting point of impure benzoic acid = Melting point of purified benzoic acid = What do your results tell you? What factors may affect the purity of your final product? _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ Evaluate the accuracy of your results ensuring that you compare your results. Do you think that your results are accurate? Can you account for any differences because of your technique? _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________ _______________________________________________________________________________