1 to 4
advertisement

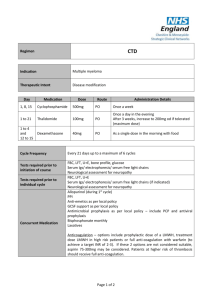
Melphalan, Prednisolone and Thalidomide Regimen Indication Multiple myeloma Therapeutic Intent Disease modification Day Medication Dose Route Administration Details 1 to 4 Melphalan 7mg/m2 PO Round to nearest 2mg 1 to 4 Prednisolone 40mg/m2 PO Once daily as a single dose in the morning 1 to 28 Thalidomide 100mg PO Once a day in the evening Increase by 50mg every 2 weeks (if tolerated) to maximum of 200mg daily Cycle Frequency Tests required prior to initiation of course Tests required prior to individual cycle Concurrent Medication Every 28 days up to a minimum of 6 cycles and a maximum of 9 cycles FBC, LFT, U+E, bone profile, glucose Serum Igs/ electrophoresis/ serum free light chains Neurological assessment for neuropathy FBC, LFT, U+E Serum Igs/ electrophoresis/ serum free light chains (if indicated) Neurological assessment for neuropathy Allopurinol (during 1st cycle) PPI Anti-emetics as per local policy GCSF support as per local policy Antimicrobial prophylaxis as per local policy – include PCP and antiviral prophylaxis Bisphosphonate monthly Laxatives Anticoagulation – options include prophylactic dose of a LMWH, treatment dose LMWH in high risk patients or full anti-coagulation with warfarin (to achieve a target INR of 2-3). If these 2 options are not considered suitable, aspirin 75-300mg may be considered. Patients at higher risk of thrombosis should receive full anti-coagulation. Page 1 of 2 Dose Modifications Hepatic No hepatic dose modifications Renal If serum creatinine >200umol/l – reduce dose of melphalan to 5mg/m2 in the first course and titrate against marrow toxicity for subsequent courses (although melphalan is hydrolysed and excreted via the kidneys, the extent of drug accumulation is variable in each individual and cannot be predicted from the degree of renal impairment) If cytopenias (i.e. neutrophils <1x109/L and platelets <75 x109/L) are considered to be chemotherapy- induced: Delay treatment for 1 to 2 weeks; delay of more than 2 weeks on more than one occasion would be an indication to give GCSF or consider a dose reduction of melphalan to 75% Add GCSF (usually only minimal dose required 2 to 3 days per cycle). Thalidomide-related grade 1-2 toxicity, but sometimes grade 3-4 toxicity, may be encountered and include constipation, neuropathy, fatigue, sedation, rash, tremor and oedema. Grade 3-4 toxicity is an indication to stop thalidomide for the remainder of the current cycle and then re-introduce at 50 mg daily with the next or subsequent cycle. Assuming tolerance at the lower dose level, escalation to 100 mg daily may be considered, and possibly to 150 mg or the full dose of 200 mg daily if the symptoms resolve and do not recur. The occurrence of a thromboembolic event such as a DVT or pulmonary embolism is an indication for full anticoagulation following standard treatment guidelines. Thalidomide may be stopped, but can be re-introduced, assuming good anticoagulant control and no other untoward side effects. Haematological Neurotoxicity Thromboembolism Additional Information Thalidomide must be supplied via an MHRA approved risk management programme only. Refer to thalidomide SPC (available at www.medicines.org.uk) for further information on thalidomide risk management programme run by Celgene. References Palumbo et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomized controlled trial. Lancet 2006; 367: 825-31. Author Pharmacy CNG Approved & Checked by Haematology CNG (Review Date = Sept 2017) Page 2 of 2