KS5 Thalidomide
advertisement

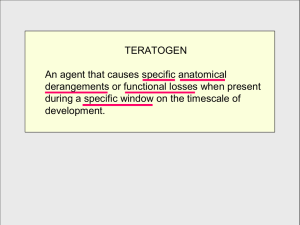
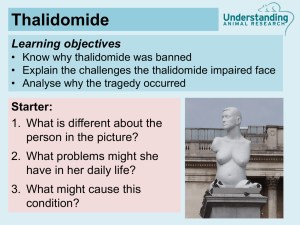
The drug ‘Thalidomide’ was introduced in Europe in the late 1950s to relieve morning sickness during pregnancy. Evidence slowly emerged that it was teratogenic, causing malformations of the foetus. Children were born with greatly shortened and distorted limbs. The thalidomide molecule is chiral. One enantiomer is teratogenic; the other alleviates morning sickness and is not teratogenic. The drug sold in Europe was a racemic mixture. US MOTHERS GIVE THANKS TO KELSEY’S Frances Kelsey was a pharmacologist who stopped the drug being used in the USA and as the human disaster unfolded she saved US mothers for the horrific birth defects witnessed in Europe. Question – Find out what the words chiral, enantiomer, teratogenic and racemic means. Birth-Defect Drug Is Back Thalidomide wins guarded approval for leprosy Thalidomide, the morning-sickness drug that became infamous for causing severe birth defects a generation ago, won FDA approval yesterday for treating leprosy patients. The FDA said its approval came with "unprecedented" restrictions. Doctors and pharmacies who prescribe the drug must register with its maker, Celgene Corp., and women must use at least two forms of birth control. Male patients have to use a condom during sex even though there's no evidence that birth defects come from sperm. "This oversight program is designed to help insure a zero-tolerance policy for thalidomide exposure during pregnancy," the agency's announcement said. Thalidomide was introduced in Europe in the late 1950s, first as a sedative and then as a remedy for morning sickness. But by 1961, scientists tied the drug to as many as 8,000 babies born with defective limbs or no limbs at all. It was quickly taken off the market in most countries, and the name of the drug became a buzzword for science gone awry. But in recent years, researchers found that thalidomide can help some very sick people, including those with leprosy (also known as Hansen's disease) and HIV. "We know a lot more about this drug than we did back then," said Dr. David Stirling, executive vice president for pharmaceutical research and development for Celgene. "It has more and more clinical benefit. The risk-benefit ratio is important, so we are only considering it for life-threatening situations." Another condition of approval is that patients clearly know that they're taking thalidomide. The name of the drug must appear prominently next to the brand name. It will be marketed under the name Thalomid. Today's approval restricts the use of the drug to treating erytema nodosum leprosum (ENL), a complication of leprosy. The inflammatory condition causes painful skin sores that, left untreated, could be fatal. The drug makes the lesions disappear quickly, Stirling said. Stirling said several thousand people in this country, mostly immigrants, suffer from leprosy. Women cannot be pregnant in order to receive the drug. If they do become pregnant, they must stop using it immediately. Men are being warned to practice safe sex, too, even though it's never shown up in semen -- but "it's tough to prove a negative," Stirling said. Clinical trials have shown thalidomide can reverse the "body-wasting" process that AIDS patients suffer from, and Celgene hopes to receive approval for that purpose sometime next year.