Dose Modifications
advertisement
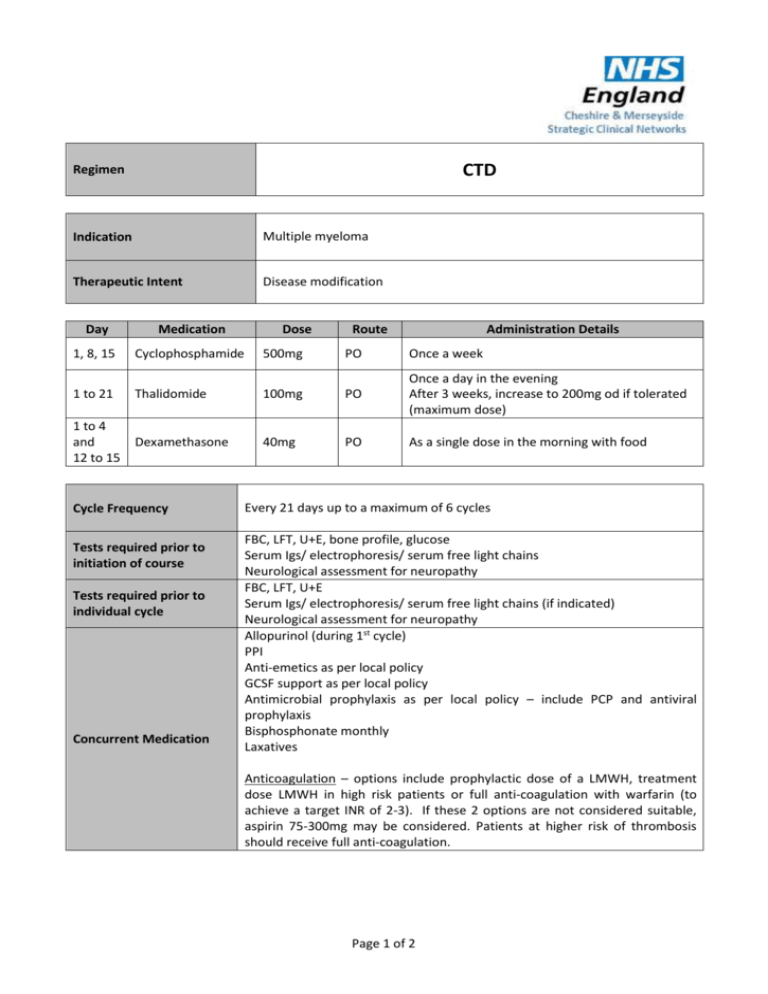
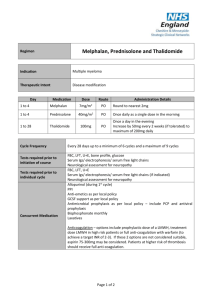
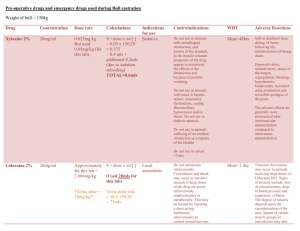
CTD Regimen Indication Multiple myeloma Therapeutic Intent Disease modification Day Medication 1, 8, 15 Cyclophosphamide 500mg PO Once a week 1 to 21 Thalidomide 100mg PO Once a day in the evening After 3 weeks, increase to 200mg od if tolerated (maximum dose) 1 to 4 and 12 to 15 Dexamethasone 40mg PO As a single dose in the morning with food Cycle Frequency Tests required prior to initiation of course Tests required prior to individual cycle Concurrent Medication Dose Route Administration Details Every 21 days up to a maximum of 6 cycles FBC, LFT, U+E, bone profile, glucose Serum Igs/ electrophoresis/ serum free light chains Neurological assessment for neuropathy FBC, LFT, U+E Serum Igs/ electrophoresis/ serum free light chains (if indicated) Neurological assessment for neuropathy Allopurinol (during 1st cycle) PPI Anti-emetics as per local policy GCSF support as per local policy Antimicrobial prophylaxis as per local policy – include PCP and antiviral prophylaxis Bisphosphonate monthly Laxatives Anticoagulation – options include prophylactic dose of a LMWH, treatment dose LMWH in high risk patients or full anti-coagulation with warfarin (to achieve a target INR of 2-3). If these 2 options are not considered suitable, aspirin 75-300mg may be considered. Patients at higher risk of thrombosis should receive full anti-coagulation. Page 1 of 2 Dose Modifications Hepatic No hepatic dose modifications Renal Haematological Neurotoxicity Thromboembolism Steroid Intolerance Serum Creatinine (micromol/L) Modification >300 Omit cyclophosphamide Dose modification is not usually indicated if cytopenias are thought be due to marrow infiltration. If neutrophil <1 x 109/l or platelets <50 x 109/l are treatment related: Omit cyclophosphamide for 1 to 3 weeks and reduce dose (e.g. to 400mg or 300mg) Add G-CSF for 2 to 3 days per cycle or week (usually only requires low doses) Thalidomide-related grade 1-2 toxicity, but sometimes grade 3-4 toxicity, may be encountered and include constipation, neuropathy, fatigue, sedation, rash, tremor and oedema. Grade 3-4 toxicity is an indication to stop thalidomide for the remainder of the current cycle and then re-introduce at 50 mg daily with the next or subsequent cycle. Assuming tolerance at the lower dose level, escalation to 100 mg daily may be considered, and possibly to 150 mg or the full dose of 200 mg daily if the symptoms resolve and do not recur. The occurrence of a thromboembolic event such as a DVT or pulmonary embolism is an indication for full anticoagulation following standard treatment guidelines. Thalidomide may be stopped, but can be re-introduced, assuming good anticoagulant control and no other untoward side effects. Patients unable to tolerate dexamethasone at the protocol dose can dose reduce e.g. 20mg daily or omit one of the two 4 day pulses of dexamethasone in a 3 week cycle. Switching to an alternative corticosteroid can also be considered. Additional Information Thalidomide must be supplied via an MHRA approved risk management programme only. Refer to thalidomide SPC (available at www.medicines.org.uk) for further information on thalidomide risk management programme run by Celgene. References Myeloma XI Trial Author Pharmacy CNG Approved & Checked by Haematology CNG (Review Date = Sept 2017) Page 2 of 2