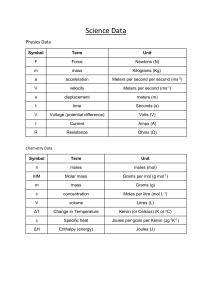
Name__________________________
advertisement

Name__________________________ Date____________________ Period________________ PRE-IB & PRE-AP CHEMISTRY 2ND 9 WEEKS FINAL EXAM REVIEW 1) In all chemical reactions, energy, mass, and charge are conserved…………T or F? ______ 2) Why is a chemical equation an example of a chemical change? 3) For the equation below, identify one substance that cannot be broken down by a chemical change and explain why – Fe(s) + O2(g) + H2O(ℓ) → Fe(OH)2(s) 4) According to the Law of Conservation of Mass and Matter, the mass of the reactants is ________________ to the mass of the products. 5) What is the activity series and what is its purpose? Balance the following chemical equations using the correct coefficients, write the word equation after it is balanced, and identify the type of reaction: 6) ____Zn (s) + ____ HCl (l) → 7) ____Mg(ClO3)2 (aq) → ____ZnCl2 (l) + ____ MgCl2 (aq) + 8) ____C (s) + ____O2 (g) → 9) ____Na (s) + ____O2 (g) → ____CO (g) Type________________________ ____O2 (g) Type ________________ Type ___________________ Type _____________________ _____Na2O (s) 10) ____Pb(NO3)2 (aq) + ____KI (aq) → ____H2 (g) Type ______________________ ____PbI2 (s) + ____KNO3 (aq) For the following questions (9-11), write the skeleton equation from the word equations, balance them with the correct coefficients and identify the type of reaction: 11) Chlorine gas reacts with potassium iodide to form a solid substance called potassium chloride and releases iodine gas. Type_____________________________ 12) When heated, solid magnesium carbonate produces a white solid magnesium oxide and releases carbon dioxide gas. Type_____________________________ 13) Sodium cyanide and copper (II) carbonate react to produce sodium carbonate and copper (II) cyanide. Type_____________________________ Find the empirical and molecular formulas for the following problems: 14) Ibuprofen, a common headache remedy, has an empirical formula of C7H9O and a molar mass of approximately 215 g/mol. 15) Epinephrine (adrenaline) is a hormone secreted into the bloodstream in times of danger and stress. It is 59.0% carbon, 7.1% hydrogen, 26.2% oxygen, and 7.7% nitrogen by mass. Its molar mass is about 180 g/mol. Solve the following molar conversions (use dimensional analysis, use correct number of sig. figs. and units to receive credit): 16. How many moles are found in 20.0 grams of H3PO4? 17. How many grams are found in 7.5 moles of BaCO3? 18. How many formula units are found in 2.0 moles of Cu(CN)2? 19. How many moles are found in 5.5 x 1022 molecules of NI3? 20. What number of formula units are found in 2.35 moles of MgO?