Topic 13 revision - PAC
advertisement
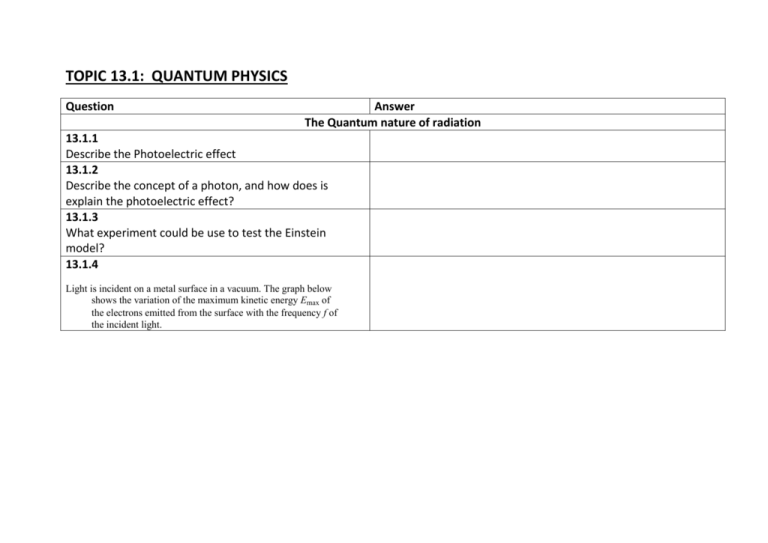
TOPIC 13.1: QUANTUM PHYSICS Question Answer The Quantum nature of radiation 13.1.1 Describe the Photoelectric effect 13.1.2 Describe the concept of a photon, and how does is explain the photoelectric effect? 13.1.3 What experiment could be use to test the Einstein model? 13.1.4 Light is incident on a metal surface in a vacuum. The graph below shows the variation of the maximum kinetic energy Emax of the electrons emitted from the surface with the frequency f of the incident light. Emax / eV 4 3 2 1 0 0 2 4 6 8 10 12 14 f / x 10 Hz –1 –2 –3 –4 (b) Use data from the graph to determine (i) the threshold frequency. (2) (ii) a value of the Planck constant. (2) (iii) the work function of the surface. (2) The threshold frequency of a different surface is 8.0 × 1014 Hz. (c) On the axes opposite, draw a line to show the variation with frequency f of the maximum kinetic energy Emax of the electrons emitted. (2) (Total 10 marks) The Wave nature of matter 13.1.5 What is the de Broglie hypothesis ? and what is mean by matter waves? 13.1.6 What experiment can be done to verify the de Broglie hypothesis? 13.1.7 (a) Explain what is meant by the de Broglie wavelength of a particle. (2) (b) Calculate the de Broglie wavelength of an electron that has been accelerated from rest through a potential difference of 5.0 kV. (4) (Total 6 marks) An electron is accelerated from rest through a potential difference of 850 V. For this electron (i) calculate the gain in kinetic energy. (1) (ii) deduce that the final momentum is 1.6 × 10–23 Ns. (2) (iii) determine the associated de Broglie wavelength. (Electron charge e = 1.6 × 10–19 C, Planck constant h = 6.6 × 10–34 J s) Atomic spectra and atomic energy states 13.1.8 What is the laboratory procedure for producing and observing atomic spectra (emission and absorption)? 13.1.9 How does the atomic spectra provide evidence for the quantization of energy in atoms, in terms of energy differences between allowed electron energy? 13.1.10 How do you calculate the wavelenghts of spectral lines from energy level differences and vice versa? 13.1.11 What is the origin of atomic energy levels in terms of the "electron in a box" model? 13.1.12 What is the Schrodinger model of the atom? 13.1.13 Discuss the Heisenberg uncertainty principle in regard to position-momentum and time-energy. TOPIC 13.2: NUCLEAR PHYSICS Question Answer Nuclear Physics 13.2.1 How can the radii of nuclei be estimated from charge particle scattering experiments? 13.2.2 How can the masses of nuclei be determined when using a Bainbridge mass spectrometer? Draw a schematic diagram of the Bainbridge spectrometer. 13.2.3 What is one piece of evidence for the existence of nuclear energy levels? Radioactive Decay 13.2.4 Describe β+ decay and include the existence of the neutrino. 13.2.5 What is the exponential fuction for radioactive decay law? What is the definition of the decay constant? 13.2.6 What is derivation of the relationship between decay constant and half-life? 13.2.7 What are the methods for measuring the half-life of an isotope? 13.2.8 The half-life of the decay of radon-222 is 3.8 days and radon-220 has a half-life of 55 s. (d) (i) Suggest three ways in which nuclei of radon-222 differ from those of radon220. (3) (ii) Define half-life. (2) (iii) State the expression that relates the activity At at time t of a sample of a radioactive material to its initial activity A0 at time t = 0 and to the decay constant λ. Use this expression to derive the relationship between the decay constant λ and the half-life T 1 . 2 mple of a radioactive material to its initial activity A0 at time t = 0 and to the decay constant λ. Use this expression to derive the relationship between the decay constant λ and the half-life T 1 . 2 (3) (iv) Radon-222 emits αparticles. The activity of radon gas in a sample of 1.0 m3 of air is 4.6 Bq. Given that 1.0 m3 of the air contains 2.6 × 1025 molecules, determine the ratio number of radon - 222 atoms in 1.0 m 3 of air number of molecules in 1.0 m 3 of air (4) (e) Suggest whether radon-222 or radon-220 presents the greater hazard to people over a long period of time. (1)










