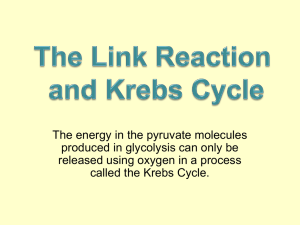
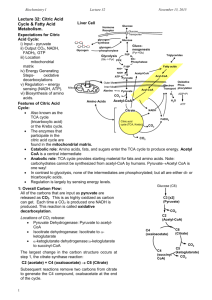
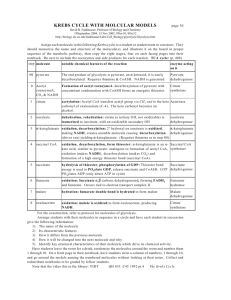
Reactions of the citric acid cycle
advertisement
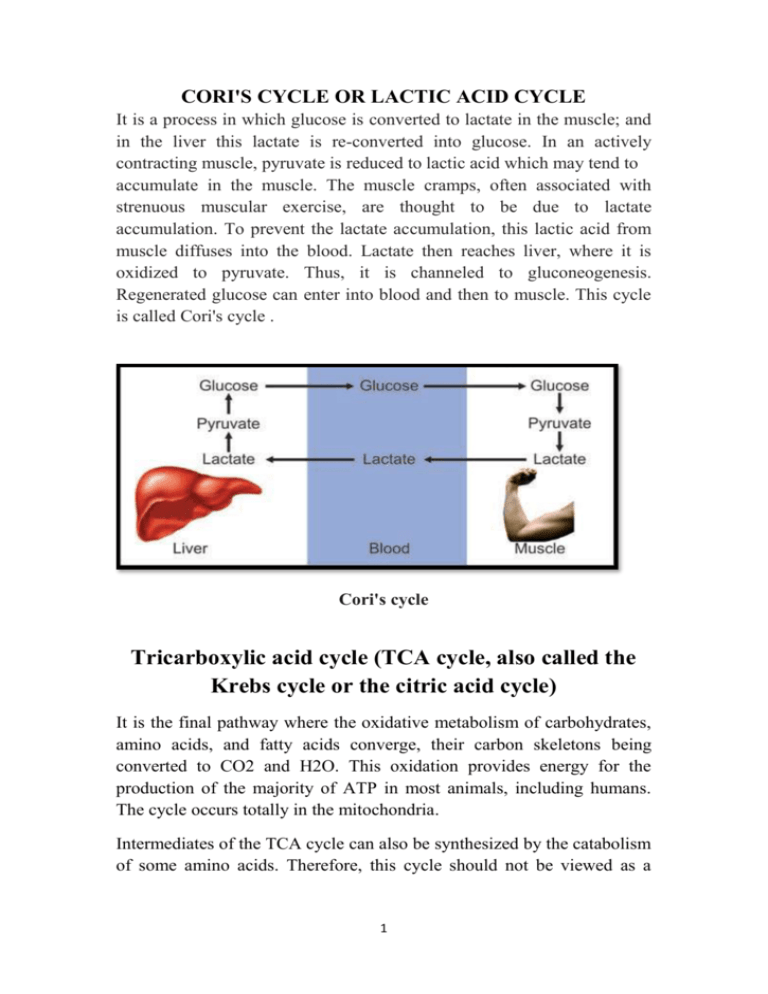
CORI'S CYCLE OR LACTIC ACID CYCLE It is a process in which glucose is converted to lactate in the muscle; and in the liver this lactate is re-converted into glucose. In an actively contracting muscle, pyruvate is reduced to lactic acid which may tend to accumulate in the muscle. The muscle cramps, often associated with strenuous muscular exercise, are thought to be due to lactate accumulation. To prevent the lactate accumulation, this lactic acid from muscle diffuses into the blood. Lactate then reaches liver, where it is oxidized to pyruvate. Thus, it is channeled to gluconeogenesis. Regenerated glucose can enter into blood and then to muscle. This cycle is called Cori's cycle . Cori's cycle Tricarboxylic acid cycle (TCA cycle, also called the Krebs cycle or the citric acid cycle) It is the final pathway where the oxidative metabolism of carbohydrates, amino acids, and fatty acids converge, their carbon skeletons being converted to CO2 and H2O. This oxidation provides energy for the production of the majority of ATP in most animals, including humans. The cycle occurs totally in the mitochondria. Intermediates of the TCA cycle can also be synthesized by the catabolism of some amino acids. Therefore, this cycle should not be viewed as a 1 closed circle, but instead as a traffic circle with compounds entering and leaving as required. Reactions of the citric acid cycle. Oxidative decarboxylation of pyruvate Pyruvate, the end-product of aerobic glycolysis, must be transported into the mitochondrion before it can enter the TCA cycle. This is accomplished by a specific pyruvate transporter that helps pyruvate cross the inner mitochondrial membrane. Once in the matrix, pyruvate is converted to acetyl CoA by the pyruvate dehydrogenase complex, which is a multienzyme complex. [Note: The irreversibility of the reaction precludes the formation of pyruvate from acetyl CoA, and explains why 2 glucose cannot be formed from acetyl CoA via gluconeogenesis.] Strictly speaking, the pyruvate dehydrogenase complex is not part of the TCA cycle proper, but is a major source of acetyl CoA— the two-carbon substrate for the cycle The Pyruvate Dehydrogenase Complex Requires Five Coenzymes— thiamine pyrophosphate (TPP), flavin adenine dinucleotide (FAD), coenzyme A (CoA, sometimes denoted CoA-SH), nicotinamide adenine dinucleotide (NAD), and lipoate. Four different vitamins required in human nutrition are vital components of this system: thiamine (in TPP), riboflavin (in FAD), niacin (in NAD), and pantothenate (in CoA). Clinical application: Pyruvate dehydrogenase deficiency: A deficiency in the pyruvate dehydrogenase complex is the most common biochemical cause of congenital lactic acidosis. This enzyme deficiency results in an inability to convert pyruvate to acetyl CoA, causing pyruvate to be shunted to lactic acid via lactate dehydrogenase .This causes particular problems for the brain, which relies on the TCA cycle for most of its energy, and is particularly sensitive to acidosis. 1. Condensation (Synthesis of citrate from acetyl CoA and oxaloacetate ) The condensation of acetyl CoA and oxaloacetate to form citrate is catalyzed by citrate synthase. 2 + Citrate synthase is allosterically activated by Ca and ADP, and inhibited by ATP, NADH, succinyl CoA, and fatty acyl CoA derivatives However, the primary mode of regulation is also determined by the availability of its substrates, acetyl CoA and oxaloacetate. [Note: Citrate, in addition to being an intermediate in the TCA cycle, provides a source of acetyl CoA for the cytosolic synthesis of fatty acids. Citrate also inhibits phosphofructokinase (PFK), the rate-limiting enzyme of 3 glycolysis, and activates acetyl CoA carboxylase (the rate-limiting enzyme of fatty acid synthesis) 2. Isomerization of citrate Citrate is isomerized to isocitrate by aconitase 3. Oxidation and decarboxylation of isocitrate Isocitrate dehydrogenase catalyzes the decarboxylation of isocitrate, yielding the molecules produced by the cycle, and the first one of the rate-limiting steps of the TCA irreversible oxidative first of three NADH release of CO2. This is cycle. The enzyme is ++ allosterically activated by ADP (a low-energy signal) and Ca , and is inhibited by ATP and NADH, whose levels are elevated when the cell has abundant energy stores. 4. Oxidative decarboxylation of α-ketoglutarate The conversion of α-ketoglutarate to succinyl CoA is catalyzed by the α-ketoglutarate dehydrogenase complex. The mechanism of this oxidative decarboxylation is very similar to that used for the conversion of pyruvate to acetyl CoA. The reaction releases the 4 second CO2 and produces the second NADH of the cycle. The coenzymes required are thiamine pyrophosphate, lipoic acid, FAD, NAD+, and coenzyme A. The equilibrium of the reaction is far in the direction of succinyl CoA—a high-energy thioester similar to acetyl CoA. a-Ketoglutarate dehydrogenase complex is inhibited by ATP, . GTP, NADH, and succinyl CoA, and activated by Ca++. 5. Cleavage of succinyl CoA Succinate thiokinase (also called succinyl CoA synthetase) cleaves the high-energy thioester bond of succinyl CoA. This reaction is coupled to phosphorylation of GDP to GTP. GTP and ATP are energetically interconvertible by the nucleoside diphosphate kinase reaction .The generation of GTP by succinate thiokinase is another example of substrate-level phosphorylation. 6. Oxidation of succinate Succinate is oxidized to fumarate by succinate dehydrogenase, producing the reduced coenzyme FADH .Succinate dehydrogenase is inhibited by oxaloacetate. 5 7. Hydration of fumarate Fumarate is hydrated to malate in a freely reversible reaction catalyzed by fumarase (also called fumarate hydratase). 8. Oxidation of malate Malate is oxidized to oxaloacetate by malate dehydrogenase. This reaction produces the third and final NADH of the cycle. REGULATION OF THE TCA CYCLE A. Regulation by activation and inhibition of enzyme activities TCA cycle is controlled by the regulation of several enzyme activities . The most important of these regulated enzymes are citrate synthase, isocitrate dehydrogenase, and ketoglutarate dehydrogenase complex. B. Regulation by the availability of ADP 1. Effects of elevated ADP: Energy consumption as a result of muscular contraction, biosynthetic reactions, or other processes results in the hydrolysis of ATP to ADP and the resulting increase in the concentration of ADP accelerates the rate of reactions that use ADP to generate ATP, most important of which is oxidative phosphorylation . Production of ATP increases until it matches the rate of ATP consumption by energy-requiring reactions. 6 2. Effects of low ADP: ADP (or Pi) is present in limiting concentration, the formation of ATP by oxidative phosphorylation decreases as a result of the lack of phosphate acceptor (ADP) or inorganic phosphate. The rate of oxidative phosphorylation is proportional to [ADP][Pi]/[ATP]; this is known as respiratory control of energy production. The oxidation of NADH and FADH2 by the electron transport chain also stops if ADP is limiting. This is because the processes of oxidation and phosphorylation are tightly coupled and occur simultaneously .As NADH and FADH2 accumulate, their oxidized forms become depleted, causing the oxidation of acetyl CoA by the TCA cycle to be inhibited as a result of a lack of oxidized coenzymes. AMPHIBOLIC PATHWAY All other pathways such as beta oxidation of fat or glycogen synthesis are either catabolic or anabolic. But TCA cycle is truly amphibolic (both catabolic and anabolic) in nature. Citric Acid Cycle components are important biosynthetic intermediates in aerobic organisms; the citric acid cycle is an amphibolic pathway, one that serves in both catabolic and anabolic processes. Besides its role in the oxidative catabolism of carbohydrates, fatty acids, and amino acids, the cycle provides precursors for many biosynthetic pathways, Ketoglutarate and oxaloacetate can, for example, serve as precursors of the amino acids aspartate and glutamate by simple transamination .Through aspartate and glutamate, the carbons of oxaloacetate and -ketoglutarate are then used to build other amino acids, as well as purine and pyrimidine nucleotides. Oxaloacetate is converted to glucose in gluconeogenesis. SuccinylCoA is a central intermediate in the synthesis of the porphyrin ring of heme groups, which serve as oxygen carriers (in hemoglobin and myoglobin) and electron carriers (in cytochromes). 7 Amphibolic pathway of the citric acid cycle Influx of TCA cycle intermediates 8 Efflux of TCA cycle intermediates Anaplerotic Reactions Anaplerotic Reactions replenish Citric Acid Cycle Intermediates as intermediates of the citric acid cycle are removed to serve as biosynthetic precursors; they are replenished by anaplerotic reactions. Under normal circumstances, the reactions by which cycle intermediates are siphoned off into other pathways and those by which they are replenished are in dynamic balance, so that the concentrations of the citric acid cycle intermediates remain almost constant. Anaplerotic Reactions are: 9