Catabolism of Carbohydrates and Fatty Acids:
advertisement

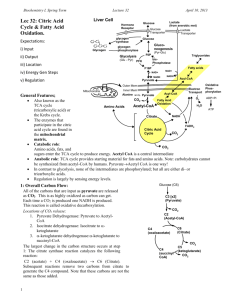
Biochemistry I Lecture 32: Citric Acid Cycle & Fatty Acid Metabolism. Lecture 32 Liver Cell Hormone Receptor November 15, 2015 Glucose Glucose Transporter Expectations for Citric Acid Cycle: glycogen Glucose synthase i) Input - pyruvate Glucoglycogen ii) Output CO2, NADH, neogenesis Glycogen phosphorylase (Pyr->Glu) FADH2, GTP F6P Triglycerides Glycolysis iii) Location bis(Glu -> Pyr) PFK Phosphatase mitochondrial Fatty acids F16P matrix NAD+ iv) Energy Generating NADH Acetyl-CoA Stepsoxidative Acyl-CoA ATP Pyruvate decarboxylations Oxidative Outer Mem v) Regulation – energy Electron PhosInner Mem Transport phorylation sensing (NADH, ATP). Mitochondria Acyl-CoA Alanine Pyruvate vi) Biosynthesis of amino ADP+Pi O2 CO2 Fatty Acid acids. Oxidation H2O Acetyl-CoA Amino Acids Features of Citric Acid ATP Cycle: NADH Citrate Also known as the TCA cycle FADH 2 Citric acidAcid (tricarboxylic acid) Citric cycle (TCA, Krebs) CO2 Cycle or the Krebs cycle. The enzymes that participate in the CO2 citric acid cycle are found in the mitochondrial matrix. Catabolic role: Amino acids, fats, and sugars enter the TCA cycle to produce energy. Acetyl CoA is a central intermediate Anabolic role: TCA cycle provides starting material for fats and amino acids. Note: carbohydrates cannot be synthesized from acetyl-CoA by humans. PyruvateAcetyl CoA is one way! In contrast to glycolysis, none of the intermediates are phosphorylated; but all are either di- or tricarboxylic acids. Regulation is largely by sensing energy levels. 1: Overall Carbon Flow: All of the carbons that are input as pyruvate are released as CO2. This is as highly oxidized as carbon can get. Each time a CO2 is produced one NADH is produced. This reaction is called oxidative decarboxylation. Locations of CO2 release: Pyruvate Dehydrogenase: Pyruvate to acetylCoA Isocitrate dehydrogenase: Isocitrate to ketoglutarate -ketoglutarate dehydrogenase:-ketoglutarate to succinyl-CoA The largest change in the carbon structure occurs at step 1, the citrate synthase reaction: C2 (acetate) + C4 (oxaloacetate) C6 (Citrate) Subsequent reactions remove two carbons from citrate to generate the C4 compound, oxaloacetate at the end of the cycle. 1 Glucose (C6) C3 [x2] (Pyruvate) CO2 C2 (Acetyl-CoA) C4 (oxaloacetate) C4 (succinyl CoA) C6 (Citrate) CO2 C5 (ketoglutarate) CO2 Biochemistry I Lecture 32 2. Energetics of the TCA Cycle: Most of the energetic currency is in the form of redox reactions, only a single ATP (GTP) is produced/pyruvate while four NADH and one FADH2 are produced. Most of the energy from oxidation is of glucose is harvested in the TCA cycle. The TCA cycle is a slower but richer source of energy. 2a: Oxidative decarboxylations: These occur at three locations, leading to the loss of the three carbons from pyruvate. 1. Pyruvate dehydrogenase (step 0) 2. Isocitrate dehydrogenase (step 3) 3. -ketoglutarate dehydrogenase (step 4) Pyruvate dehydrogenase (decarboxylase)(Step 0) 1. loss of the CO2 group. 2. oxidation of the aldehyde and formation of the thio-ester. (The thio-ester is the same oxidation state as a carboxylate.) The thio-ester is formed between the oxidized product and Coenzyme A, to form acetyl-CoA. November 15, 2015 Glucose F6P bisPhosphatase-1 PFK-1 F 1,6 P 2 ATP 2 NADH Pyr NAD+, CoA CO2 NADH Oxaloacetate [Pyruvate 0 dehydrogenase] Acetyl-CoA Citrate CoA 8 [citrate synthase] 1 Go=-31 kJ/mol 2 NADH Malate iso-citrate 8 NADH =24 ATP 2 FADH2= 4 ATP 2 GTP = 2 ATP 7 Fumarate FADH2 [succinate 6 dehydrogenase] ATP GTP NADH [isocitrate 3 dehydrogenase] NADH CoA 4 Succinate [a-ketoglutarate dehydrogenase] a-ketoglutarate [succinate 5 thiokinase] succinyl-CoA NADH CO2 NAD+ Pyruvate Acetyl-CoA coenzymeA (CoA) Thioesters in Biochemical Reactions: The relatively weak thioester bond facilitates the transfer of the attached group to other compounds. i) Citrate synthase mechanism (Step 1). Asp-375 – general base His-274 – general acid A) proton abstraction by Asp375, proton donation by His274 B) nucleophilic attack of –ene to C=O on oxaloacetate. C) hydrolysis of thioester. A Oxaloacetate Citrate B C 1 3 3 2 2 1 2 HS-CoA Biochemistry I Lecture 32 A B November 15, 2015 C Succinate ii) Succinate thiokinase (Step 5): succinyl CoA can provide enough energy to driving the synthesis of GTP. GTP A) phosphorlysis of thioSuccinyl-CoA CoA ester. B) Transfer of phosphate to His C) Transfer from phosphoryl-His to GDP, forming GTP. S-CoA GDP 2b. The remaining section of the pathway, from succinate to oxaloacetate follows a classic three step oxidation scheme (also seen in fatty acid oxidation): Alkane → Alkene → Alcohol → Ketone REDOX REDOX Step 6. Oxidation of succinate to fumarate reduces FAD to FADH2. Alkane → Alkene O FAD OH O O O CH3 N HN CH3 N N OH O H N CH3 CH3 N N H COH COH COH CH2 O O P NH2 O O N N O P H O O 2 C O N N H H OR HO O OH Succinate HN COH COH COH 2e- + 2 H+ CH2 O O P NH2 O O N N O P H O O 2 C O N N FADH2 HH HH O O OH Fumarate HO Step 7. Addition of water to the double bond, to make the alcohol. Alkene → Alcohol OR FADH2 (Reduced) FAD (Oxidized) Glucose PFK-1 Fructose-6-P bisPhosphatase-1 Fructose-1,6-P Malate Fumarate O OH NAD+ NADH O CO2 O [Pyruvate dehydrogenase] NAD+, CoA OH NADH CH2 O O H3C H HO O Pyr Step 8. Oxidation of Malate to Oxaloacetate reduces NAD+ to NADH. Alcohol → Ketone O OH CH2 O O OH Oxaloacetate Malate Oxaloacetate O C C O OH O Acetyl-CoA C CoA H3C S CoA O C OH [Citrate synthase] HO OH C C COOH C Citrate Regulation of the TCA Cycle: O OH 1. High energy, irreversible steps are regulated. 2. Regulated reactions are at the "top" of the Succinyl-CoA pathway. Examples of: 1. Product Inhibition. 2. Allosteric inhibition by feedback inhibition by products 'downstream' in the pathway. Energy sensing is the most important regulatory control of the TCA cycle (I=inhibited) High Energy OTHER Step NADH ATP Compound Product Inh Feedback Inh I I Pyruvate Inhibited by Acetyl Co-A Dehydrogenase COOH CH2 CH2 O Citrate Synthase I I S-CoA Inhibited by succinyl-CoA Inhibited by citrate Regulation of glycolysis: Citrate stabilizes the tense-form of PFK, shutting down glycolysis. 3 Biochemistry I Lecture 32 November 15, 2015 Fatty Acid Oxidation (-Oxidation): A. Formation of Acyl-CoA: Length N FAD 1 FADH2 AMP 2ATP 2ADP 2 H2O Acyl-CoA (Cytosol): Additional The fatty acids in the Cycles 5 NAD+ cytosol are coupled 3 to coenzyme A to NADH form acyl-CoA. The activation reaction is catalyzed by acylCoenzyme A CoA synthetase and 4 involves the hydrolysis of ATP to AMP, i.e. the N-2 carbons equivalent of two (shrinking chain) high energy ATP molecules (60 kJ/mol). The released pyrophosphate is hydrolyzed to inorganic phosphate, making the overall ΔG negative for the reaction (indirect coupling). Note: it is only necessary to utilize ATP once in the activation of the fatty acid. B. Transport into mitochondria: The acyl-CoA is transported into the mitochondrial matrix -ideal for funneling the products of -oxidation (NADH and FADH2) to E. transport. C. -Oxidation (Mito. matrix): Acyl-CoA is shortened 2 carbons at a time from the carboxyl end of the fatty acid using the following steps: 1. Formation of trans - double bond by acyl-CoA dehydrogenase, an FAD enzyme. 2. Addition of water to the newly formed double bond to generate the alcohol by enoyl-CoA hydratase 3. Oxidation of the alcohol by NAD+ to give the ketone, catalyzed by 3-L-hydroxyacyl-CoA dehydrogenase. 4. Cleavage reaction by -ketoacy-CoA thiolase (thiolysis), generates acetyl-CoA and an acylCoA that is two carbons shorter. The acetyl-CoA enters the TCA cycle. 5. Steps 1-4 are repeated until only acetyl-CoA remains. The last cycle produces two acetylCoA. H3C O O Oxidation of C6 Fatty Acid H3C O O S CoA H3C CoA S O S CoA CoA H3C H3C O O S CoA O CoA O S H3C S CoA H3C S CoA CoA Fatty Acid Synthesis: Occurs in the cytosol using acetyl CoA (derived from citrate), elongating by 2 carbons at a time, each pair of Cs is added from malonyl-CoA. Electron donor is NADPH. Malonyl-S Acetyl-CoA 1 Acetyl-CoA 4 2 1 3 2 3