Objectives 11
advertisement

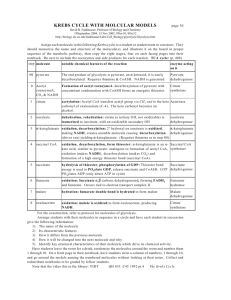
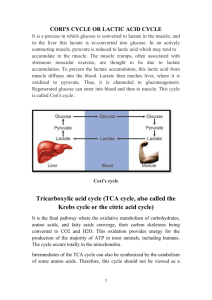
1. - in fed state pyruvate produced from glycolysis (glucose) - in fed state 2/3 of carbon from pyruvate directed to fat synthesis for fuel storage (in adipose cells and lesser extent in liver) - aerobic muscle and heart pyruvate completely oxidized to CO2 for energy - pyruvate can be produced from alanine via alanine aminotransferase and from lactate via lactate dehydrogenase; this occurs in liver where pyruvate is the metabolized to glucose (gluconeogenesis: pyruvate carboxylated oxaloacetate via pyruvate carboxylase) - when pyruvate used for energy production or fatty acid biosynthesis pyruvate undergoes oxidative decarboxylation to acetyl CoA via pyruvate dehydrogenase in mitochondrial matrix - pyruvate needs specific transporter to cross inner mitochondrial membrane (passes easily across outer membrane) - Acetyl CoA generated in mitochondrial matrix from pyruvate, beta-oxidation of fatty acids (not in nervous tissue or erythrocytes) or oxidation of ketone bodies (not in liver or erythrocytes) - one fate of Acetyl CoA is its complete oxidation to CO2 in citric acid cycle - other fates of acetyl CoA 1.) synthesis of fatty acids during fed state, primarily in liver and adipose cells; fatty acids stored as TAG in adipose cells 2.) formation of ketone bodies, during starvation, via ketogenesis in liver or in insulindependent diabetics who have missed insulin injection 3.) production of cholesterol in liver 4.) formation of steroid hormones via cholesterol in endocrine glands (adrenal cortex, testes, ovaries) 2. Pyruvate dehydrogenase pyruvate + NAD + CoA acetyl CoA + NADH + H+ + CO2 E1: pyruvate binds to E1 component and reacts with TDP prosthetic group causing carbon dioxide to be released; remaining 2-C unit stays attached to TDP and serves as a substrate for E2; thiamine is the vitamin precursor for TDP E2 : contains oxidized lipoate (-S-S-) as a prosthetic group which is covalently attached to a lysine side chain creating a lipoamide group; lipoate oxidizes the 2-C fragment from E1, and reacts this product with the sulfhydryl group of coenzyme A (CoASH) to form the acetyl CoA product; thus TDP is regenerated on E1 for the next reaction, while the lipoate on E2 becomes reduced (-SH SH-); pantothenic acid is the vitamin precursor for coenzyme A; lipoic acid is not a vitamin derivative E3: FAD on the E3 component reoxidizes lipoate to regenerate (-S-S-). The now reduced flavin group (FADH2) is reoxidized by the coenzyme NAD+ NADH as a product. Riboflavin is the vitamin precursor for FAD/FADH2 and niacin is the vitamin precursor for NAD+/NADH table on 11-3 3. Regulation of pyruvate dehydrogenase (PDH) complex by allostery and by covalent modification Allosteric regulation - PDH allosterically inhibited by acetyl CoA (E2 component) and NADH (E3 component) - NADH provides electrons for respiratory (electron transport) chain (oxidative phosphorylation) - enough ATP NADH accumulates in mitochondrial matrix shuts off E3 enzyme to prevent mitochondria from unnecessarily continuing to oxidize pyruvate to acetyl CoA (which would result in depletion of free coenzyme A supplies) - when mitochondria oxidizing fats large production of NADH and Acetyl CoA both these inhibit the additional production of these substances from pyruvate metabolism Regulation of PDH by covalent modification - reaction inhibited when E1 is phosphorylated by PDH kinase; removal of phosphate (activation) catalyzed by PDH phosphatase; both of these are regulated - products of PDH reaction (acetyl CoA and NADH) allosterically activate PDH kinase phosphorylation of PDH-E1 inhibition; substrates of PDH reaction (pyruvate, CoASH, and NAD) and ADP allosterically inhibit PDH kinase prevents phosphorylation of PDHE1 active form of PDH; - when energy low (high ADP) keeping PDH active will favor oxidation of pyruvate production of energy - Ca2+ (released during muscle contraction) allosterically controls this process by inhibiting PDH kinase and by activating PDH phosphatase; increased energy demands during muscle contraction met by sustaining flux through PDH 4. Citric acid cycle reactions associated with energy production (formation of GTP, NADH, FADH2)/identify substrate, cofactor, and vitamin source of cofactors Citrate synthase (no energy required) -located in matrix - condensation of Acetyl CoA with oxaloacetate citrate - CoA produced as a product produces acetyl CoA via PDH - spontaneously initiates cycle Isocitrate dehydrogenase - Isocitrate alpha-ketoglutarate (NAD NADH, CO2) - oxidation-reduction reaction - Isocitrate is reductant and NAD is oxidant (Isocitrate is oxidized to alphaketoglutarate/reduces NADNADH); step releases CO2 (1st of 2 carbons lost in cycle) - first site of NADH production yields 3 ATP via oxidative phosphorylation Alpha-ketoglutarate dehydrogenase - alpha-ketoglutarate succinyl CoA (NAD, CoA NADH, CO2) - oxidation-reduction reaction - alpha-ketoglutarate is reductant and NAD is oxidant - CO2 produced in step (2nd of 2 carbons lost in cycle) - NADH formed 3 more ATP in ox-phos - mechanism similar to PDH (no regulation by covalent modification); succinyl CoA is an allosteric inhibitor Succinyl CoA synthetase - succinyl CoA succinate (GDP GTP, ADP ATP) - substrate level phosphorylation - uses energy for the formation of succinyl CoA - the GTP formed transfers its high energy phosphate to ADP forming ATP via nucleoside diphosphokinase; GTP + ADP GDP + ATP) Succinate dehydrogenase - succinate fumarate (FAD FADH2) - enzyme attached to inner membrane of mitochondria - oxidation-reduction reaction - FAD is oxidant; succinate is reductant; FADH2 formation generates 2 ATP via ox-phos Malate dehydrogenase - malate oxaloacetate (NAD NADH) - oxidation-reduction - NAD is electron acceptor/get reduced (oxidant) NADH 3 ATP - regenerates oxaloacetate needed to restart cycle 5. Allosteric regulation of Citric Acid cycle - when respiration and synthesis of ATP slows NADH accumulates allosteric inhibition by NADH of isocitrate dehydrogenase and alpha-ketoglutarate dehydrogenase along with pyruvate dehydrogenase - therefore accumulation of NADH is prevented and depletion of NAD is prevented - ATP and GTP (high energy levels) inhibit cycle at isocitrate dehydrogenase (ATP) and alpha-ketoglutarate dehydrogenase (GTP) - low energy levels (high ADP) ADP allosterically activates isocitrate dehydrogenase - if flux through 2nd half of cycle (succinate oxaloacetate) diminishes succinyl CoA accumulates and causes feedback inhibition of both alpha-ketoglutarate dehydrogenase and citrate synthase to shut down 1st half of cycle (oxaloacetate succinate)