Standardization of NaOH
advertisement

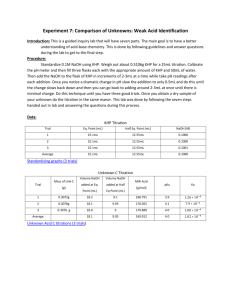
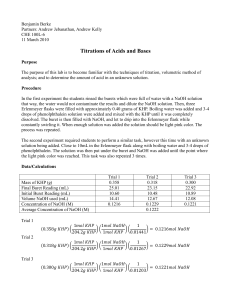
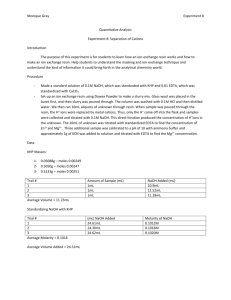
THE STANDARIZATION OF NaOH USING KHP THEORY: The General Issue This is a lab in which we must recognize that (quite simply) chemists do not always assume that good enough is … good enough. Sometimes knowing a concentration as precisely as is possible, is very important. We are charged with learning how to determine as precise a concentration as possible or how to standardize a solution of NaOH (aq) It is surprisingly difficult to make a solution of NaOH, and to know its precise concentration. You think you have made a 0.20 M solution of NaOH (aq), but in all probability, you haven’t. Most times “good enough” is fine … but not always. Sodium hydroxide has a number of very practical problems. First, NaOH solid is not only hygroscopic (it picks up water from the air) …but it is also deliquescent (in that it picks up so much water, it dissolves itself) even while it is sitting on the balance! This process of absorbing water ruins even the most precise student’s efforts as s/he attempts to mass a sample of NaOH, due to the added mass of water. Thus we can’t trust the mass value. Secondly, (and such a bother), sodium hydroxide absorbs carbon dioxide from the air as well! This produces sodium carbonate. Thus knowing the absolute concentrations of sodium hydroxide solutions can be challenging. What do chemists mean by, standardization of a base? Standardization of a substance (such as NaOH)(aq), means to determine a precise and accurate concentration. You can standardize almost any strong base solution and almost any strong acid solution (although that is a bit more involved). Two general methods of analysis are available to us, in our lab. We can use gravimetric analysis or volumetric analysis. You are most familiar with gravimetric analysis, in which we isolate the analyte (that species which we desire to analyze) in a solid (such as a precipitate) and we then mass the precipitate. Every time we filter, dry the residue and determine the residue, it is essentially a gravimetric analysis. This mass may lead us to a mole value … and then we are off … to working out such issues as; empirical formulae, molecular formulae, percent compositions, melting points, solubilities etc… However, to accomplish the standardization of NaOH (aq) we shall use the OTHER method of analysis available to us … volumetric analysis. Volumetric analysis may use (but is not limited to the use of) volume to determine the concentration of an analyte in an aqueous solution. This is most commonly done via titration. The Concept of a Titration and of a Primary Standard, such as KHP We will run an acid/base titration in this experiment (as opposed to another type of titration, called a redox titration). An acid-base titration is a method used to compare the number of moles of acid in one sample with the number of moles of base in a second sample. In this experiment we will try to master the technique to standardize (or to determine the exact concentration) of a sample of NaOH. To do this we need to react the NaOH solution with a primary standard. Thus, titration is a common methodology used to carry out a volumetric analysis. Standardization, via titration essentially determines the concentration of a sample by reacting it with a primary standard. A primary standard is an ultra-pure, and reasonably chemically stable compound, such as; oxalic acid dihydrate or potassium hydrogen phthalate (That last word, phthalate, is pronounced as thāl-āte) The requirements for a primary standard are that it:3 1) 2) 3) 4) has a high purity (99.% or so pure) remains unchanged in air during massing and remain stable during storage possesses a high molar mass to reduce massing errors reacts with the solution to be standardized in a direct, well-defined reaction In this experiment, we will react a solution of NaOH having an approximate 0.10 M concentration, with potassium hydrogen phthalate (abbreviated as KHP). Titration and the Use of an Indicator, Such as Phenolphthalein Since this is an acid-base titration, we will need to use an indicator. As a rule, acids and base solutions are colorless. To see the changes, in concentrations, we use a chemical which can react to changes in [H+] or [OH-]. We will use phenolphthalein (fē-nol-thāl-ēn … abbreviated as phth). Phth is clear in solutions with a pH <8.2, but light pink to dazzling fuchsia-violet-pink in solutions with pH >8.2 or so. Indicators are weak acids and weak bases, they are in equilibrium with their molecular and ionized form. When acid is added to the indicator the equilibrium shifts to the left. The In-1 and HIn have different colors (due to structural changes, which alter the quantum absorption & reflection of light). We can see this change in color. When a base is added to an indicator the hydroxide ions reacts with the molecules of indicator and form indicator ions. The reaction shifts to the right. OH-1(aq) + HIn(aq) In-1(aq) + H2O(l) Since different indicators have different Ka values (or Kb values) they changes colors at different [H+1], and they can be used to indicate the hydrogen-ion concentration in a solution. A single indicator will tell us only whether the [H+] is greater than, less than, or about the same as its Ka value. You select the indicator according to the pH where you would like to see a color change. For example, phenolphthalein has a pKa of 9.2. We would expect to see a color change in a solution having a pH of about 9. The two forms of phenolphthalein are shown here.7 Deprotonation change …changing bonding O OH Deprotonation change Deprotonation change HO C OH O C CO CO O O Colorless Red As base is added, the phth deprotonates, and the structural changes, alter the activity in light, reflecting pink. Potassium Hydrogen Phthalate: KHP Note that there is no phosphorus in KHP. The “P” stands for the word phthalate …The formula for KHP is KHC8H4O4. It has a mole mass of 204.23 g/mol KHP acts as a weak acid in solution … It will react with NaOH in a 1:1 stoichiometric ratio … remember this!!!!!! KHP dissociates completely in water, giving the potassium cation (K+) and hydrogen phthalate anion (HP- or Hphthalate-, HC8H4O4-). The hydrogen phthalate anion is really the weak acid and it produces an equilibrium with water to give hydronium (H3O+) and phthalate ions (C8H4O42-). HP- + H2O The structure of KHP is: P2- + H3O+ O COH CO K O When KHP and a base a reacted, a neutralization reaction occurs that is represented by: KHC8H4O4 (aq) + NaOH(aq) KNaC8H4O4 (aq) + H2O(l) The net ionic equation is: HC8H4O4-1(aq) + OH-(aq) C8H4O4-2 (aq) + H2O(l) The reaction can be considered to proceed completely to the right. When exactly equivalent amounts of acid or base are used so that neither reactant is present in excess the solution is said to be at the equivalence point. When only monoprotic acids and bases are used (those that furnish or react with one H+ per molecule), then at the equivalence point the number of moles of acid equal the number of moles of base (moles acid = moles base).6 To analyze the role of the primary standard, KHP in this standardization reaction, study the following diagrams.7 204.23 g/mol Note that the acidic hydrogen of the KHP is from a carboxylic group (-COOH) In terms of the “net ionic” equation we can visualize the prior diagram as: KHP is essentially an acid in both the Bronsted-Lowry sense and the Lewis sense. (Recall all B-L acids are Lewis acids, but not all Lewis acids are B-L acids) It is a Bronsted-Lowry acid, in that the carboxyl group donates (acids donate) a H+. This can conversely be interpreted as Lewis acid activity, in that we might say that the H+ from KHP accepts an electron pair from the OH- of the base…. Just a bit of review…. Okay? You see, all of this standardization goes to the fact that we want to use the NaOH for a purpose. For instance, in an acid-base titration, it is critical to know the exact concentration of the titrant (the solution in the burette which will be reacted, drop wise, with the unknown) in order to determine the concentration of the solution being tested … Thus if we wished to know the exact molar concentration in a vinegar solution, we must know the concentration of the NaOH solution we shall add to it … The first step then is to standardize the NaOH with something like KHP, and to then apply any remaining standardized solution to testing the vinegar. BEFORE YOU COME TO LAB, a few calculations should be completed. Essentially we need to know how much KHP you need to use … So calculate how many: a) milliliters of 6.0 M NaOH are required to create a diluted solution of 250 mL of 0.10 M NaOH. b) moles of NaOH there are in 25.0 mL of 0.10 M NaOH ( a convenient volume to use in a burette) c) moles of KHP you will need to react with the moles of NaOH you calculated in calculation b). Recall that the reading indicated the stoichiometric relationship … d) grams of KHP corresponds to the number of moles of KHP you calculated in calculation c). Recall that the reading provided the molecular formula for KHP as well as the molar mass of KHP. Diagram of General Set-Up: 2 edited burette an organic clamp or a butterfly clamp will work …for this part of the lab, the burette holds NaOH(aq) …for this part of the lab, this has the KHP distilled water and phth a yellow or white card to help read the burette a piece of white paper CLEANING / STANDARDIZING / FILLING THE BURETTE: 1) Gather together the required materials: 1-buret, 1-butterfly clamp, 4-125 mL flasks, 1-250 mL beaker 1-pipette and bulb, 1-funnel , 2-index cards (or pieces of colored paper), a wash bottle with distilled water Put on your safety goggles, this is no time to be goofing around, you are using a BASE! 2) Bring your 250 mL flask with you. Under the hood you will find a solution of approximately 6.0 M NaOH. Shake the stoppered flask carefully. 6.0 M NaOH is dense and tends to sit on the bottom of the flask, while less dense water sits on top. When the NaOH solution is not well mixed, your experiment WON’T WORK. Make sure the cap is on tightly and invert the bottle of solution at least 30 times. Using the graduated cylinder under the hood, measure the required volume (from calculation “a”) of your prelab calculations. Pour this aliquot into a 250 mL volumetric flask, and add distilled water, so as to dilute the solution to 0.10 M NaOH 3) Obtain and clean a 50.00 mL burette and ring stand. Rinse it and Drain it several times with distilled water. Each time you rinse it, make sure you open the stopcock and run distilled water through the tip. When the burette is clean, it may have some water in it. You don’t want to dilute your NaOH solution, so now… 4) Rinse your burette with your diluted NaOH solution. Carefully pour about 5 mL of your well-mixed NaOH solution into your burette (Make sure the stopcock is closed!). At one of the 2 large sinks, open the stopcock and drain a little solution through the tip. Close the stopcock. Now, tilt your burette down to near horizontal, twirl it to coat the walls with solution, and then discard the rinse solution. Repeat this process 2 to 3 more times. Now, return to your bench and; 5) Fill your burette with your dilute NaOH solution. Fill the burette to close to the top, but DO NOT fill to the 0.00 line. This introduces error! Rarely is this done correctly, and essentially, it is your worst reading. Don’t waste time trying to get to 0.00 mL … just fill and read the burette, as instructed. 6) BLEED your burette …thus filling the tip with base. 7) Read your burette volume & record it. This is you INITIAL reading of NaOH. Remember to read the bottom of the meniscus, with your sight-line level with the meniscus. A white or yellow card behind the burette will help you take more accurate readings. Record the volume to 0.01 mL. 4 8) Get your 4 125 mL flasks The flasks can be labeled as, “1, 2, 3, and 4. Use the labeling tape or a wax pencil. The flasks need to be clean, but not necessarily dry (really!) That’s because the amount of water in which the KHP is dissolved, doesn’t matter … since we are determining the number of moles of the KHP acid, and not the molarity of the acid. Think about that…. 9) Mass a sample of KHP to 0.0001 g required to react with 25 mL of 0.10 M NaOH you calculated before you came to lab (calculation d). You can find the KHP in the oven. It has been dried overnight at 120oC. Place mass of KHP you have measured out, in the 125 mL Erlenmeyer flask labelled “1”. 9) Add about 10 mL of distilled water and swirl it about. It’s perfectly fine, were some KHP to remain undissolved.. (It will dissolve as it reacts, as you titrate with NaOH). Set this first labeled flask aside and go prepare 3 more labelled flasks by massing out 3 more samples of KHP. Record the mass of each.…. 10) Add 2 drops of phenolphthalein to the KHP mixture in each of your 4 flasks. Swirl the flasks. DOING THE TITRATION: Do at least 4 trials … and take the average of your results. 1) Get your first labeled flask with the known and recorded mass of KHP. (Be sure 2 drops of phenolphthalein indicator have been added to it.) Situate that flask beneath the burette with the tip inside the flask. Make sure the burette tip has no drops dangling from the tip. If it does, rinse the tip with distilled water from your wash bottle into a waste beaker. Have you recorded your burette volume? If not do it now, to the nearest 0.01 mL. 2) Start adding NaOH. Slowly open the stopcock and start running NaOH into your flask. You know the approximate volume required so you might save some time by letting the titrant run … Rinse down the sides of the flask from time to time with your wash bottle. Swirl the mixture gently … swirling is important (If we had enough magnetic stirrers, we could use those) Wash down the sides of your flask while titrating. Soon, you will see pink color forming around the drops of NaOH as they interact with the solution. SWIRL the flask!!!!! The pink color will rapidly disappear. 3) As the pink colors seems to linger, Slow Down! As you move toward the endpoint, the pink color will fade more slowly [This is the collision theory in action Kiddos … As the concentration of reactants decreases (as in H+), there are fewer effective collisions, thus the rate of the reaction slows!]. Begin to add one drop at a time. Try spinning the stopcock to generate a ½ drop. Rinse the sides and tip of the burette with distilled water from your wash bottle. As you go on, add NaOH slower. When the faint pink blush of phenolphthalein lasts for > 30 seconds, you’re done. You should be able to hit this endpoint to +/- ½ drop. Save your flask so you can match the faint pink color with your next endpoint. Take a photo if you wish … label it. 4 That persistent faint pink blush of a good endpoint. Ugh! You overshot the end point … go slowly when you are in doubt! 4) Record your burette reading +/- 0.01 mL. 5) Repeat steps 1-4 with your other 3 flasks of previously massed and recorded KHP. Name____________________________________ STANDARDIZATION OF NaOH USING KHP Data Table for the Standardization of NaOH(aq) with KHP Trial 1 Trial 2 Trial 3 Trial 4 Mass of KHP (g) Initial burette reading (mL) Final burette reading (mL) Volume of NaOH used (mL) Volume of NaOH used (L) Moles of KHP Moles of NaOH Molarity of NaOH Average Molarity of NaOH This is a classic approach to standardizing a solution of NaOH. At this point we can now go back and titrate a number of vinegar samples, so as to determine the molarity of the vinegar…..(but now, with standardized base!) Annotated Bibliography 1. Chemtech.org: http://www.chemtech.org/cn/cn1105/experiments/standardization_NaOH.pdf 2. Hanna T, : Cornell University: CSIP: Cornell Science Inquiry Partnership: The Standardization of NaOH Solution http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=3&ved=0CC4QFjAC&url=http%3A%2F%2Fcsip.cornell. edu%2FCurriculum_Resources%2FCSIP%2FHanna%2FTHanna_studentVersion.doc&ei=XUyvVMC1A3gsASA8oHoCw&usg=AFQjCNHGWhn5Yeo6FOuP0O50GtEYyQqUNQ&bvm=bv.83339334,d.cWc 3. Rushin, W.G. http://chem.lapeer.org/Chem2Docs/AcidBaseTitration.html 4. Spurlock, D., Indiana University Southeast: http://homepages.ius.edu/dspurloc/c121/week11.htm 5. Standardization of NaOH: http://www.foothill.edu/attach/psme/holland.1astdnaohlab.pdf 6. Standardization of NaOH with KHP: www.dlt.ncssm.edu/core/.../KHP_Stand_of_NaOH_web_version.doc 7. St. Michael’s College: Standardizing your NaOH academics.smcvt.edu/ http://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=8&ved=0CEkQFjAH&url=http%3A%2F%2Facademics.s mcvt.edu%2Fchemistry%2FCHEM_103%2FCHEM_103%2FCHEM_103_Labs%2FAspirin%2FSTANDARDIZING%2520YOU R%2520NaOH.doc&ei=d06vVL-tK4nIsATBmoK4Ag&usg=AFQjCNFE53bl5KZZTxocy5Zh9nRtLvzUg&bvm=bv.83339334,d.cWc 8. Wikipedia: edited; http://en.wikipedia.org/wiki/Potassium_hydrogen_phthalate