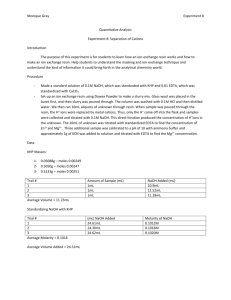
Titrations of Acids and Bases
Benjamin Berke
Partners: Andrew Jebanathan, Andrew Kelly
CHE 100L-6
11 March 2010
Titrations of Acids and Bases
Purpose
The purpose of this lab is to become familiar with the techniques of titration, volumetric method of analysis; and to determine the amount of acid in an unknown solution.
Procedure
In the first experiment the students rinsed the burets which were full of water with a NaOH solution that way, the water would not contaminate the results and dilute the NaOH solution. Then, three
Erlenmeyer flasks were filled with approximately 0.40 grams of KHP. Boiling water was added and 3-4 drops of phenolphthalein solution were added and mixed with the KHP until it was completely dissolved. The buret is then filled with NaOH, and let to drip into the Erlenmeyer flask while constantly swirling it. When enough solution was added the solution should be light pink color. The process was repeated.
The second experiment required students to perform a similar task, however this time with an unknown solution being added. Close to 10mL in the Erlenmeyer flask along with boiling water and 3-4 drops of phenolphthalein. The solution was then put under the buret and NaOH was added until the point where the light pink color was reached. This task was also repeated 3 times.
Data/Calculations
Mass of KHP (g)
Final Buret Reading (mL)
Initial Buret Reading (mL)
Volume NaOH used (mL)
Concentration of NaOH (M)
Average Concentration of NaOH (M)
Trial 1
Trial 1
0.358
25.01
10.60
14.41
0.1216
Trial 2
0.318
23.15
10.48
12.67
0.1229
0.1222
Trial 3
0.300
22.92
10.89
12.08
0.1221
1𝑚𝑜𝑙 𝐾𝐻𝑃
(0.358𝑔 𝐾𝐻𝑃) (
204.2𝑔 𝐾𝐻𝑃
) (
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1𝑚𝑜𝑙 𝐾𝐻𝑃
1
) (
0.01441
) = 0.1216𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
Trial 2
1𝑚𝑜𝑙 𝐾𝐻𝑃
(0.318𝑔 𝐾𝐻𝑃) (
204.2𝑔 𝐾𝐻𝑃
) (
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1𝑚𝑜𝑙 𝐾𝐻𝑃
1
) (
0.01267
) = 0.1229𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
Trial 3
1𝑚𝑜𝑙 𝐾𝐻𝑃
(0.300𝑔 𝐾𝐻𝑃) (
204.2𝑔 𝐾𝐻𝑃
) (
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1𝑚𝑜𝑙 𝐾𝐻𝑃
1
) (
0.01203
) = 0.1221𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
Standard Deviation
√
(0.1216 − 0.1222) 2 + (0.1229 − 0.1222) 2 + (0.1221 − 0.1222) 2
2
= 6.557 𝑋 10 −3
Volume of Unknown (mL)
Final Buret Reading (mL)
Initial Buret Reading (mL)
Volume NaOH used (mL)
Concentration of Unknown (M)
Average Concentration of Unknown (M)
Trial 1
9.00
23.32
10.80
12.52
0.1699
Trial 2
9.18
24.01
10.46
13.55
0.1802
0.1770
Trial 1
(0.1252𝐿) (
0.1221𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
) (
1𝐿
1𝑚𝑜𝑙 𝐾𝐻𝑃
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1
) (
0.0900
) = 0.1699 𝑀𝑜𝑙 𝐾𝐻𝑃
Trial 2
(0.1355𝐿) (
0.1221𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
) (
1𝐿
1𝑚𝑜𝑙 𝐾𝐻𝑃
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1
) (
0.0918
) = 0.1802 𝑀𝑜𝑙 𝐾𝐻𝑃
Trial 3
(0.1364𝐿) (
0.1221𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
) (
1𝐿
1𝑚𝑜𝑙 𝐾𝐻𝑃
1𝑚𝑜𝑙 𝑁𝑎𝑂𝐻
1
) (
0.0920
) = 0.1810 𝑀𝑜𝑙 𝐾𝐻𝑃
Standard Deviation
Trial 3
9.20
24.02
10.38
13.64
0.1810
√
(0.1699 − 0.1770) 2 + (0.1802 − 0.1770) 2 + (0.1810 − 0.1770) 2
2
= 6.19 𝑋10 −3
Results
Trial 1
Molarity of NaOH 0.1216 M
NaOH
Molarity of KHP 0.1699 M
KHP
Trial 2
0.1229 M
NaOH
0.1802 M
KHP
Trial 3 Average
0.1221 M NaOH 0.1222 M
NaOH
0.1810 M KHP 0.1770 M
KHP
Standard Devation
6.557 X 10
6.19 X 10 -3
-3
The averages for both parts of the experiment were not supposed to exceed 0.1 M, and with the averages of the molarities of NaOH being 0.1222 M and the average molarities of KHP being 0.1770 M, the results can be said to be fairly accurate.
Conclusion
The experiment was a success because the standard deviation was with within acceptable range.