Lab 4 Report
advertisement

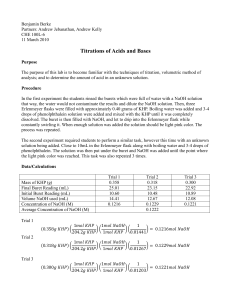
Experiment 4 pre-lab Ken McFarland “Strong acid / Strong base titrations”, CHEM 1130, TA - Nastaran Marzijarani, Section 108, Room 1871, Will Brubacher, Thursday 2:00 pm 1. Purpose of Experiment: This experiment is to determine the exact concentration of a sodium hydroxide solution, then use that solution to determine the acid content of car battery fluid. 2. Procedure: Materials: 25 mL buret, volumetric pipettes, 250 mL volumetric flask, KHP, 6.00 M NaOH, dilute battery acid, bromothymol blue indicator, Prepare a 250 mL 0.100 M NaOH solution, by dilution, using a volumetric flask. When this solution has been prepared, rinse, and then fill, the buret with it. Calculate the amount of KHP required to reach equilibrium, and measure it out into a clean 125 mL Erlenmeyer flask. Add 25 mL of DI water, and 4 drops of bromothymol blue to the flask. Titrate the solution in the flask, making sure to record the initial and final buret readings. The solution in the flask will turn blue when equilibrium is reached. Repeat at least 2 more times, until you have 3 trials that all agree to within +/- 0.40 mL of each other. Calculate an amount of battery acid that will not require more than 25 mL of NaOH to titrate. Check your work with your lab instructor. Obtain enough of the battery acid for each member of your group to do the titration once. To titrate it, pipette the amount needed into a 125 mL Erlenmeyer flask, and add enough water to make a total solution of 40 mL, and 4 drops of bromothymol blue. Titrate until the solution turns blue, same as the KHP solution. All of the solutions in this lab can be rinsed down the drain with water. 3. Observations, Results and Data Sheets: See attached data sheet. 4. Error Analysis: The battery acid may not have been diluted to exactly a 5:1 ratio, which could cause error in either direction, depending on whether the acid was diluted too much or too little. The KHP could also have impurities in it, which would lead to the amount of KHP in the solution being less than the calculated amount. This would cause the calculated molarity of the NaOH solution to be too high, as it would take less of the solution to titrate the calculated, incorrect, amount of KHP. 5. Questions: 1. Our RSD was 1.8%. This is higher than the standard of 1.0%. Therefore our trials were much less precise than they ideally should have been. The differences in the amount of NaOH solution used for each trial were greater than they should have been, leading to a RSD that is higher than the norm. 2. To insure that the exact concentration of the NaOH solution is known, so the concentration of the battery acid solution can be accurately found. 3. The equivalence point is when the amount of the titrant added is equal to the amount of analyte in the solution being titrated, while the end point is the point when the indicator in the solution changes color. 4. The battery acid was strong enough that it had to be diluted to facilitate titrating it, if it had not been diluted it would of taken much more NaOH to titrate it, or a much more concentrated NaOH solution. The concentrated acid is also much more dangerous, as would be an NaOH solution concentrated enough to easily titrate it. 5. 2PbSO4(s) + 2H2O(l) → PbO2(s) + Pb(s) + 2H2SO4(aq)