Exp. 15: Volumetric Analysis: Total Hardness of Water by EDTA
advertisement

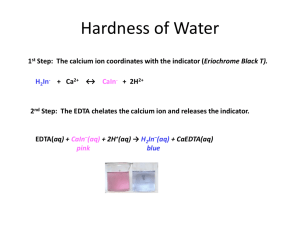
Exp. 15 – video (time: 21:39 minutes) Exp. 15: Volumetric Analysis: Total Hardness of Water by EDTA Hardness – is defined in terms of the capacity of cations in the water to replace the sodium or potassium ions in soaps and form sparingly soluble products (insoluble). • Most multiple charged cations (>+1) • Natural waters – most abundant Ca2+ and Mg2+ Hardness for most part is expressed in terms of the concentration of calcium and magnesium in the sample. Why is hardness important? Measure of quality of water for household and industrial use (hard waters can precipitate calcium carbonate which can clog filters, pipes, etc.) Water Hardness is typically determined by an EDTA titration buffered at a pH of 10 (condition for maximum complexing). EDTA – ethylenediaminetetraacetic acid (1:1 mol ratio between divalent metal (Ca2+, Mg2+) and EDTA) There is an order of stability of metal complexes formed with EDTA. Calcium tends to form a more stable complex with EDTA than magnesium. This means if Ca2+ is present in a water sample, the calcium EDTA complex will form before any Mg2+ forms a complex with EDTA How will we determine eq pt? Ca2+ - eriochrome black T complex – dark red (maroon) uncomplexed black T – blue color one drop red blue (lack of red tint) EDTA is standardized with primary standard CaCO3 To simplify calculations with hardness we use CaCO3 titer = mg CaCO3 mL EDTA After EDTA is standardized, we can use the ratio to determine the hardness of a water sample by titrating with EDTA and using the CaCO3 titer. Total Hardness is typically reported in ppm CaCO3 ppm -parts per million ppm CaCO3 = mg CaCO3 L water sample aq: 1ppm = 1mg 1L Standardization Start experiment on page 104 - 2nd paragraph of Standardization (skip blank run) Changes: Use 10.00 mL of 0.01000 M CaCO3 (Standard solution) instead of 25 mL. 2 mL pH 10 buffer instead of 5 mL 10 mL deionized water instead of 25 mL 4-6 drops black T indicator (note: add indicator from start of titration) Do a minimum of 3 runs – want good precision avg titer ± s mg CaCO3 mL EDTA end pt approx 8-12 mL Standardization Calculation Goal: mg CaCO3 mL EDTA Note: molarity of EDTA not required Unknown Water Titration Do experiment as written except add indicator from start and try to make first run count (don’t do rapidly). Do a minimum of 3 runs – want good precision Unknown end pt approx 5-12 mL avg ppm ± s ppm CaCO3 of unknown water sample Also must report based on the water hardness scale the classification of your unknown water sample. soft, slightly hard, moderately hard, hard, very hard (must find scale on own and submit in report discussion) Unknown Water Sample Calculation Goal: ppm CaCO3 = mg CaCO3 L H2O sample Amount of chemicals to obtain in small beaker per group: EDTA – 150 mL pH buffer – 40 mL (keep under hood until you need it) 0.01000 M CaCO3 standard solution – 50 mL Unknown water – 200 mL Additional question: Hard water is a major issue in which science is used solve the problem. After your conclusion in exp 15’s lab report, you will discuss another major issue in which science is involved: Climate change is thought to be accelerated by carbon dioxide from coal and natural gas fueled power plants. Due to the increasing need for electricity, other power sources will be required to lessen the emissions from fossil fuels. List three possible alternative sources for the production of electricity. For two of your responses, describe an advantage and disadvantage between it and current coal and natural gas power plants.