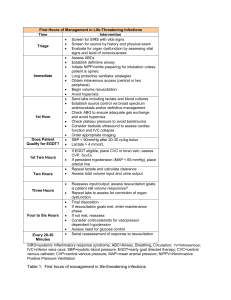
A SYSTEMATIC REVIEW AND META
advertisement

A SYSTEMATIC REVIEW AND META-ANALYSIS OF EARLY GOAL-DIRECTED THERAPY FOR SEPTIC SHOCK: THE ARISE, ProCESS AND ProMISe INVESTIGATORS SUPPLEMENTARY MATERIALS TABLE OF CONTENTS PAGE 1. THE ARISE, PROCESS, PROMISE AND PRISM INVESTIGATORS 2 2. SEARCH STRATEGY 3 3. INCLUDED STUDIES 4 4. SUPPLEMENTARY FIGURES 5 Figure S1. Random-effects model for primary mortality outcome for patients presenting to the emergency department with septic shock Figure S2. In-hospital mortality Figure S3. 28-day mortality Figure S4. Receipt and duration of invasive mechanical ventilation Figure S5. Receipt and duration of vasopressors Figure S6. Receipt and duration of renal replacement therapy Figure S7. Hospital length of stay Figure S8. Effect of EGDT on primary mortality outcome for patients with septic shock irrespective of presenting source Figure S9. Funnel plot for primary mortality outcome of individual studies 5. SUPPLEMENTARY TABLES 17 Table S1. Receipt and duration of organ support Table S2. ICU admission and length of stay in ICU and hospital Table S3. Quality assessment for included studies 6. ACKNOWLEDGEMENTS 20 1 1. The ARISE, ProCESS, ProMISE and PRISM Investigators ARISE Investigators: S.L. Peake, A. Delaney, M. Bailey, R. Bellomo, P.A. Cameron, D.J. Cooper, A.M. Higgins, A. Holdgate, B.D. Howe, S.A.R. Webb, P. Williams; ProCESS Investigators: D.M. Yealy, J.A. Kellum, D.T. Huang, P.C. Hou, A.E. Barnato, L.A. Weissfeld, F. Pike, T. Terndrup, H.E. Wang, F. LoVecchio, M Filbin, N.I. Shapiro, D.C. Angus; ProMISe Investigators: D. Bell, J.F. Bion, T.J. Coats, R.D. Grieve, D.A. Harrison, S.E. Harvey, R. Jahan, P.R. Mouncey, T.M. Osborn, G.S. Power, K.M. Rowan, M.Z. Sadique, M. Singer, J. Tan, J.D. Young; PRISM Investigators: M.C. Reade. Clinical Trials Unit, Intensive Care National Audit & Research Centre, London (J.Tan); Burns, trauma and critical care research centre, University of Queensland, Brisbane, QLD and Joint Health Command, Australian Defence Force, Canberra, ACT (M.C.Reade). All other investigator affiliations and a full list of site investigators for the ARISE, ProCESS and ProMISE trials are available in the original study reports: ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P (2014). Goaldirected resuscitation for patients with early septic shock. N Engl J Med 371:1496-506 ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC (2014). A randomised trial of protocol-based care for early septic shock. N Engl J Med 370:1683-93 Mouncey PR, Osborn TM, Power, GS, Harrison DA, Sadique MZ, Grieve RG, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM, ProMISe Trial Investigators (2015). Trial of early, goal-directed resuscitation for septic shock. N Engl J Med, doi:10.1056/NEJMoa1500896 2 2. SEARCH STRATEGY Medline via PUBMED interface (central venous oxygen saturation) OR (goal directed therapy) OR (goal directed resuscitation) OR (ScvO2) OR ScvO2) OR (clinical protocol) OR (sepsis protocol) OR (EGDT) OR (early goal directed therapy) AND (sepsis) OR (septicaemia) OR (septic shock)) AND (clinical[Title/Abstract] AND trial[Title/Abstract]) OR clinical trials[MeSH Terms] OR clinical trial[Publication Type] OR random*[Title/Abstract] OR random allocation[MeSH Terms] OR therapeutic use[MeSH Subheading]) Searched from1/1/2000 until 15/1/2015 EMBASE and Cochrane Central Register of Clinical Trials via Ovid interface 1. exp *sepsis/ 2. septicemia.mp. 3. exp Shock, septic/ 4. 1 or 2 or 3 5. early goal directed therapy.mp. 6. EGDT.mp. 7. sepsis protocol.mp. 8. exp clinical protocols/ 9. scvo2.mp. 10. goal directed resuscitation.mp. 11. goal directed therapy.mp. 12. 5 or 6 or 7 or 8 or 9 or 10 or 11 13. central venous oxygen saturation.mp. 14. 12 or 13 15. random:.tw. 16. clinical trial:.mp. 17. exp health care quality/ 18. 15 or 16 or 17 19. 4 and 14 and 18 20. limit 19 to human 21. limit 20 to yr="2000-Current" Searched from 1/1/2000 until 15/1/2015 3 3. INCLUDED STUDIES Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M; Early Goal-Directed Therapy Collaborative Group (2001). Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 8;345:1368-77 Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators (2010). Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomised clinical trial. JAMA 303:739-46 ProCESS Investigators, Yealy DM, Kellum JA, Huang DT, Barnato AE, Weissfeld LA, Pike F, Terndrup T, Wang HE, Hou PC, LoVecchio F, Filbin MR, Shapiro NI, Angus DC (2014). A randomised trial of protocol-based care for early septic shock. N Engl J Med 370:1683-93 ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, Delaney A, Bailey M, Bellomo R, Cameron PA, Cooper DJ, Higgins AM, Holdgate A, Howe BD, Webb SA, Williams P (2014). Goaldirected resuscitation for patients with early septic shock. N Engl J Med 371:1496-506 Mouncey PR, Osborn TM, Power, GS, Harrison DA, Sadique MZ, Grieve RG, Jahan R, Harvey SE, Bell D, Bion JF, Coats TJ, Singer M, Young JD, Rowan KM, ProMISe Trial Investigators (2015). Trial of early, goal-directed resuscitation for septic shock. N Engl J Med, doi:10.1056/NEJMoa1500896 de Oliveira DS, Gottschald AF, Moura JD, Costa GA, Ventura AC, Fernandes JC, Vaz FA, Carcillo JA, Rivers EP, Troster EJ (2008). ACCM/PALS haemodynamic support guidelines for paediatric septic shock: an outcomes comparison with and without monitoring central venous oxygen saturation. Intensive Care Med 34:1065-75 Wang X, Lu CJ, Gao FQ, Li XH, Yan WF, Ning FY (2006). Efficacy of goal-directed therapy in the treatment of septic shock. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 18:661-4 Early Goal-Directed Therapy Collaborative Group of Zhejiang Province (2010). The effect of early goaldirected therapy on treatment of critical patients with severe sepsis/septic shock: a multi-centre, prospective, randomised, controlled study. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 22:331-4 Tian HH, Han SS, Lv CJ, Wang T, Li Z, Hao D, Shang QM, Wang XZ (2012). The effect of early goal lactate clearance rate on the outcome of septic shock patients with severe pneumonia. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 24:42-5 Yu B, Tian HY, Hu ZJ, Zhao C, Liu LX, Zhang Y, Zhu GJ, Wang LT, Wu XH, Li J (2013). Comparison of the effect of fluid resuscitation as guided either by lactate clearance rate or by central venous oxygen saturation in patients with sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25:578-83 Lu N, Zheng R, Lin H, Shao J, Yu J (2014). Clinical studies of surviving sepsis bundles according to PiCCO on septic shock patients. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 26:23-7 4 4. SUPPLEMENTARY FIGURES Figure S1. Random-effects model of primary mortality outcome for patients presenting to the emergency department with septic shock Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 38/130 59/133 13.06 Jones et al. (2010) 1.47 (0.82, 2.60) 34/150 25/150 11.09 ProCESS Investigators (2014) 1.17 (0.88, 1.55) 92/439 167/902 23.74 ARISE Investigators (2014) 0.98 (0.76, 1.26) 147/792 150/796 25.79 ProMISe Investigators (2015) 1.02 (0.80, 1.30) 184/623 181/620 26.32 Overall (I-squared = 56.7%, p = 0.055) 0.99 (0.79, 1.25) 495/2134 582/2601 100.00 NOTE: Weights are from random effects analysis .3 1 Favours EGDT 3 Favours control EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval. The control was usual care or another nonEGDT resuscitation strategy. Random-effects model. The individual points denote the OR of each study and the lines either side the 95% confidence intervals. The vertical line denotes the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 5 Figure S2. In-hospital mortality for patients presenting to the emergency department with septic shock A. Fixed-effect model Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 38/130 59/133 11.21 Jones et al. (2010) 1.47 (0.82, 2.60) 34/150 25/150 5.25 ProCESS Investigators (2014) 1.17 (0.88, 1.55) 92/439 167/902 23.47 ARISE Investigators (2014) 0.91 (0.69, 1.20) 115/793 125/797 28.95 ProMISe Investigators (2015) 1.05 (0.81, 1.36) 160/625 154/625 31.12 Overall (I-squared = 59.2%, p = 0.044) 1.00 (0.87, 1.16) 439/2137 530/2607 100.00 Events, Events, % .3 1 Favours EGDT 3 Favours control B. Random-effects model Study ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 38/130 59/133 13.87 Jones et al. (2010) 1.47 (0.82, 2.60) 34/150 25/150 11.89 ProCESS Investigators (2014) 1.17 (0.88, 1.55) 92/439 167/902 24.01 ARISE Investigators (2014) 0.91 (0.69, 1.20) 115/793 125/797 24.57 ProMISe Investigators (2015) 1.05 (0.81, 1.36) 160/625 154/625 25.66 Overall (I-squared = 59.2%, p = 0.044) 0.98 (0.77, 1.25) 439/2137 530/2607 100.00 NOTE: Weights are from random effects analysis .3 1 Favours EGDT 3 Favours control EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval. The control was usual care or another nonEGDT resuscitation strategy. The individual points denote the OR of each study and the lines either side the 95% confidence intervals. The vertical lines denote the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 6 Figure S3. 28-day mortality for patients presenting to the emergency department with septic shock A. Fixed-effect model Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.32, 0.87) 40/130 61/133 11.57 ProCESS Investigators (2014) 1.09 (0.83, 1.44) 103/405 197/827 26.76 ARISE Investigators (2014) 0.91 (0.70, 1.20) 117/792 127/797 29.90 ProMISe Investigators (2015) 1.02 (0.79, 1.32) 155/625 152/621 31.77 Overall (I-squared = 54.7%, p = 0.085) 0.95 (0.82, 1.10) 415/1952 537/2378 100.00 .3 Favours EGDT 1 Favours control 2 B. Random-effects model Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.32, 0.87) 40/130 61/133 14.31 ProCESS Investigators (2014) 1.09 (0.83, 1.44) 103/405 197/827 28.03 ARISE Investigators (2014) 0.91 (0.70, 1.20) 117/792 127/797 28.19 ProMISe Investigators (2015) 1.02 (0.79, 1.32) 155/625 152/621 29.47 Overall (I-squared = 54.7%, p = 0.085) 0.92 (0.73, 1.15) 415/1952 537/2378 100.00 NOTE: Weights are from random effects analysis .3 Favours EGDT 1 Favours control 2 EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval. The control was usual care or another nonEGDT resuscitation strategy. The individual points denote the OR of each study and the lines either side the 95% confidence intervals. The vertical lines denote the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 7 Figure S4. Receipt and duration of invasive mechanical ventilation for patients presenting to the emergency department with septic shock A. Receipt of invasive mechanical ventilation (fixed-effect model) Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 72/130 94/133 8.18 Jones et al. (2010) 0.85 (0.54, 1.34) 69/150 75/150 7.99 ProCESS Investigators (2014) 1.17 (0.92, 1.48) 165/434 307/892 24.59 ARISE Investigators (2014) 0.93 (0.76, 1.16) 238/793 251/798 34.57 ProMISe Investigators (2015) 1.02 (0.80, 1.31) 179/620 175/615 24.67 Overall (I-squared = 55.0%, p = 0.064) 0.97 (0.86, 1.10) 723/2127 902/2588 100.00 Events, Events, % .3 1 Favours EGDT 2 Favours control B. Receipt of invasive mechanical ventilation (random-effects model) Study ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 72/130 94/133 10.99 Jones et al. (2010) 0.85 (0.54, 1.34) 69/150 75/150 12.89 ProCESS Investigators (2014) 1.17 (0.92, 1.48) 165/434 307/892 24.96 ARISE Investigators (2014) 0.93 (0.76, 1.16) 238/793 251/798 26.86 ProMISe Investigators (2015) 1.02 (0.80, 1.31) 179/620 175/615 24.31 Overall (I-squared = 55.0%, p = 0.064) 0.93 (0.77, 1.14) 723/2127 902/2588 100.00 NOTE: Weights are from random effects analysis .3 1 Favours EGDT 2 Favours control 8 C. Duration of invasive mechanical ventilation (days) (fixed-effect model) Study N, mean N, mean % (SD); EGDT (SD); control Weight ID WMD (95% CI) Rivers et al. (2001) 0.00 (-3.73, 3.73) 72, 9 (11.4) ProCESS Investigators (2014) -0.90 (-2.56, 0.76) 165, 6.4 (8.4) 307, 7.3 (9.4) 24.43 ARISE Investigators (2014) 1.50 (0.18, 2.82) 238, 6.2 (9) ProMISe Investigators (2015) 0.40 (-1.04, 1.84) 179, 6.6 (7.1) 175, 6.2 (6.7) 32.48 Overall (I-squared = 40.2%, p = 0.171) 0.48 (-0.34, 1.30) 723 -4 Favours EGDT 0 Favours control 94, 9 (13.1) 4.81 251, 4.7 (5.4) 38.28 902 100.00 4 EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval; WMD, weighted mean difference; SD, standard deviation. The control was usual care or another non-EGDT resuscitation strategy. The individual points denote the OR or WMD of each study and the lines either side the 95% confidence intervals. The vertical lines denote the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 9 Figure S5. Receipt and duration of vasopressors for patients presenting to the emergency department with septic shock A. Receipt of vasopressors (fixed-effect model) Study Events, Events, % ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.56 (0.34, 0.92) 48/130 68/133 9.08 Jones et al. (2010) 1.29 (0.79, 2.10) 108/150 100/150 6.00 ProCESS Investigators (2014) 1.06 (0.84, 1.34) 269/439 540/902 29.32 ARISE Investigators (2014) 1.67 (1.34, 2.08) 605/793 525/798 26.57 ProMISe Investigators (2015) 1.25 (1.00, 1.57) 377/623 344/625 29.04 Overall (I-squared = 78.9%, p = 0.001) 1.25 (1.10, 1.41) 1407/2135 1577/2608 100.00 Events, Events, % .3 1 Favours EGDT 3 Favours control B. Receipt of vasopressors (random-effects model) Study ID OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.56 (0.34, 0.92) 48/130 68/133 15.10 Jones et al. (2010) 1.29 (0.79, 2.10) 108/150 100/150 15.10 ProCESS Investigators (2014) 1.06 (0.84, 1.34) 269/439 540/902 23.04 ARISE Investigators (2014) 1.67 (1.34, 2.08) 605/793 525/798 23.46 ProMISe Investigators (2015) 1.25 (1.00, 1.57) 377/623 344/625 23.30 Overall (I-squared = 78.9%, p = 0.001) 1.15 (0.86, 1.53) 1407/2135 1577/2608 100.00 NOTE: Weights are from random effects analysis .3 1 Favours EGDT 3 Favours control 10 C. Duration of vasopressors (days) (fixed-effect model) Study N, mean N, mean % ID WMD (95% CI) (SD); EGDT (SD); control Weight Rivers et al. (2001) -0.50 (-1.83, 0.83) 48, 1.9 (3.1) 68, 2.4 (4.2) 2.46 ProCESS Investigators (2014) 0.10 (-0.13, 0.33) 269, 2.6 (1.6) 540, 2.5 (1.6) 79.23 ARISE Investigators (2014) 0.10 (-0.39, 0.59) 605, 2.6 (4.9) 525, 2.5 (3.4) 18.31 Overall (I-squared = 0.0%, p = 0.682) 0.09 (-0.12, 0.29) 1407 1577 -2 Favours EGDT 0 Favours control 100.00 2 EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval; WMD, weighted mean difference; SD, standard deviation. The control was usual care or another non-EGDT resuscitation strategy. The individual points denote the OR or WMD of each study and the lines either side the 95% confidence intervals. The vertical lines denote the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 11 Figure S6. Receipt and duration of renal replacement therapy for patients presenting to the emergency department with septic shock A. Receipt of renal replacement therapy Study Events, Events, % ID OR (95% CI) EGDT control Weight ProCESS Investigators (2014) 0.71 (0.36, 1.37) 12/382 35/796 11.88 ARISE Investigators (2014) 0.99 (0.74, 1.31) 106/793 108/798 50.39 ProMISe Investigators (2015) 1.09 (0.79, 1.51) 88/620 81/614 37.73 Overall (I-squared = 0.0%, p = 0.517) 0.99 (0.81, 1.22) 206/1795 224/2208 100.00 .3 Favours EGDT 1 Favours control 2 B. Duration of renal replacement therapy (days) Study N, mean N, mean % Weight ID WMD (95% CI) (SD); EGDT (SD); control ProCESS Investigators (2014) -1.50 (-8.86, 5.86) 12, 7.1 (10.8) 35, 8.6 (12.4) 3.55 ARISE Investigators (2014) -0.50 (-5.16, 4.16) 106, 8.5 (17.1) 108, 9 (17.7) 8.85 ProMISe Investigators (2015) -0.30 (-1.78, 1.18) 88, 4.8 (5.5) 81, 5.1 (4.3) 87.60 Overall (I-squared = 0.0%, p = 0.950) -0.36 (-1.75, 1.03) 206 224 100.00 -10 Favours EGDT 0 Favours control 10 EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval; WMD, weighted mean difference; SD, standard deviation. The control was usual care or another non-EGDT resuscitation strategy. Fixed-effect model. The individual points denote the OR or WMD of each study and the lines either side the 95% confidence intervals. The vertical line denotes the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 12 Figure S7. Hospital length of stay for patients presenting to the emergency department with septic shock (days) Study N, mean N, mean % ID WMD (95% CI) (SD); EGDT (SD); control Weight Rivers et al. (2001) 0.20 (-3.12, 3.52) 130, 13.2 (13.8) 133, 13 (13.7) 7.38 Jones et al. (2010) 0.70 (-1.86, 3.26) 150, 12.1 (11.7) 150, 11.4 (10.9) 12.48 ProCESS Investigators (2014) -0.70 (-1.90, 0.50) 439, 11.1 (10) 902, 11.8 (11.5) 56.68 ARISE Investigators (2014) -1.00 (-3.66, 1.66) 793, 14.1 (17.3) 797, 15.1 (34.2) 11.50 ProMISe Investigators (2015) 1.10 (-1.51, 3.71) 625, 17.6 (23) 621, 16.5 (24) 11.96 Overall (I-squared = 0.0%, p = 0.650) -0.28 (-1.18, 0.62) 2137 2603 100.00 -4 0 Favours EGDT 4 Favours control EGDT denotes early goal-directed therapy; WMD, weighted mean difference; CI, confidence interval; SD, standard deviation. The control was usual care or another non-EGDT resuscitation strategy Fixed-effect model. The individual points denote the WMD of each study and the lines either side the 95% confidence intervals. The vertical line denotes the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. 13 Figure S8. Effect of EGDT on primary mortality outcome for patients with septic shock irrespective of presenting source A. Fixed-effect model by sub-group according to presenting source Study Events, Events, % OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 38/130 59/133 8.57 Jones et al. (2010) 1.47 (0.82, 2.60) 34/150 25/150 4.01 ProCESS Investigators (2014) 1.17 (0.88, 1.55) 92/439 167/902 17.95 ARISE Investigators (2014) 0.98 (0.76, 1.26) 147/792 150/796 25.30 ProMISe Investigators (2015) 1.02 (0.80, 1.30) 184/623 181/620 26.55 Subtotal (I-squared = 56.7%, p = 0.055) 1.01 (0.88, 1.16) 495/2134 582/2601 82.38 Wang et al. (2006) 0.48 (0.11, 2.11) 4/16 7/17 1.06 de Oliveira et al. (2008) 0.21 (0.07, 0.57) 6/51 20/51 3.66 EGDT Collaborative (2010) 0.46 (0.28, 0.74) 41/163 64/151 10.33 Tian et al. (2012) 3.55 (1.15, 10.99) 12/19 14/43 0.66 Yu et al. (2013) 1.56 (0.42, 5.78) 7/25 5/25 0.75 Lu et al. (2014) 1.06 (0.34, 3.35) 7/40 7/42 1.17 Subtotal (I-squared = 72.2%, p = 0.003) 0.61 (0.43, 0.86) 77/314 117/329 17.62 0.94 (0.82, 1.07) 572/2448 699/2930 100.00 ID Source: ED . Source: other/unclear . Overall (I-squared = 70.7%, p = 0.000) .1 1 Favours EGDT 10 Favours control 14 B. Random-effects model by sub-group according to presenting source Study Events, Events, % OR (95% CI) EGDT control Weight Rivers et al. (2001) 0.52 (0.31, 0.86) 38/130 59/133 11.20 Jones et al. (2010) 1.47 (0.82, 2.60) 34/150 25/150 10.26 ProCESS Investigators (2014) 1.17 (0.88, 1.55) 92/439 167/902 14.59 ARISE Investigators (2014) 0.98 (0.76, 1.26) 147/792 150/796 15.03 ProMISe Investigators (2015) 1.02 (0.80, 1.30) 184/623 181/620 15.14 Subtotal (I-squared = 56.7%, p = 0.055) 0.99 (0.79, 1.25) 495/2134 582/2601 66.22 Wang et al. (2006) 0.48 (0.11, 2.11) 4/16 7/17 3.17 de Oliveira et al. (2008) 0.21 (0.07, 0.57) 6/51 20/51 5.56 EGDT Collaborative (2010) 0.46 (0.28, 0.74) 41/163 64/151 11.66 Tian et al. (2012) 3.55 (1.15, 10.99) 12/19 14/43 4.83 Yu et al. (2013) 1.56 (0.42, 5.78) 7/25 5/25 3.86 Lu et al. (2014) 1.06 (0.34, 3.35) 7/40 7/42 4.70 Subtotal (I-squared = 72.2%, p = 0.003) 0.77 (0.35, 1.68) 77/314 117/329 33.78 0.87 (0.65, 1.17) 572/2448 699/2930 100.00 ID Source: ED . Source: other/unclear . Overall (I-squared = 70.7%, p = 0.000) NOTE: Weights are from random effects analysis .1 1 Favours EGDT 10 Favours control EGDT denotes early goal-directed therapy; OR, odds ratio; CI, confidence interval. The control was usual care or another nonEGDT resuscitation strategy. The individual points denote the OR of each study and the lines either side the 95% confidence intervals. The vertical lines denote the null effect. The control for the ProCESS trial includes both usual care and protocol-based standard therapy groups combined. Post-hoc analysis of the interaction between presenting source (emergency department versus general ward/ intensive care unit or source unknown) and the effect of EGDT did not find any sub-group interaction (P=0.51) 15 SE of log(OR) Figure S9. Funnel plot for primary mortality outcome of individual studies Primary Secondary 0.0 0.2 0.4 0.6 0.8 0.2 0.5 1.0 Odds ratio 2.0 5.0 The dots represent those studies conducted in patients presenting to the emergency department with septic shock (primary objective) and the triangles those studies in which some or all the patients enrolled were from the general ward and /or intensive care unit or the source was unable to be determined from either the published article or from direct communication with the author (secondary objective). 16 5. SUPPLEMENTARY TABLES Table S1. Receipt and duration of organ support Author Invasive Duration of Vasopressor Duration of ventilation, % ventilation, days support, % vasopressors, days EGDT Control EGDT Control EGDT Control RRT, % Duration of RRT, days EGDT Control EGDT Control EGDT Control Primary objective Rivers et al. 55.4 70.7 9.0 ± 11.4 9.0 ± 13.1 36.1 51.1 1.9 ± 3.1 2.4 ± 4.2 NA NA NA NA Jones et al. 46.0 50.0 NAa NAa 72.0 66.7 NA NA NA NA NA NA ProCESS Investigators 38.0 34.4 6.4 ± 8.4 8.4 ± 7.3 61.3 59.9 2.6 ± 1.6 2.5 ±1.6 3.1 4.4 7.1 ± 10.8 8.6 ± 12.4 ARISE Investigators 30.1 31.5 6.2 ± 9.0 4.7 ± 5.4 76.6 65.8 2.6 ± 4.9 2.5 ± 3.4 13.4 13.6 8.5 ± 17.1 9.0 ± 16.9 ProMISe Investigators 28.9 28.5 6.6 ± 7.1 6.2 ± 6.7 60.5 55.0 NA NA 14.2 13.2 4.8 ± 5.5 5.1 ± 4.3 Secondary objective Wang et al. 68.8 76.5 NA NA 100 100 NA NA 18.8 29.4 NA NA De Oliviera et al. 70.6 80.4 NAb NAb 72.6 74.5 NAb NAb NA NA NA NA EGDT collaborative NA NA 13.2 ± 1.5 14.4 ± 1.6 NA NA NA NA NA NA NA NA Tian et al. NA NA 6.4 ± 4.0 5.7 ± 4.2c NA NA NA NA NA NA NA NA Yu et al. 76.0 80 NA NA 64.0 64 NA NA NA NA NA NA Lu et al. 100 100 6.0 ± 1.2 4.1± 0.9 100 100 NA NA NA NA NA NA Data presented as mean ± SD or proportions (%) unless otherwise indicated. ProCESS denotes Protocolized Care for Early Septic Shock; ARISE, Australasian Resuscitation in Sepsis Evaluation; ProMISe, Protocolised Management in Sepsis; EGDT, early goal-directed therapy; NA, not available; RRT, renal replacement therapy. The primary objective included only those studies in which patients presented to the emergency department with septic shock. The secondary objective included those studies in which the presenting source was the emergency department and the ward or intensive care unit or where the source was not known. The control for all analyses was usual care or another non-EGDT resuscitation strategy. For ProCESS, data presented for the control group is usual care and protocol-based standard therapy groups combined. a Ventilator-free days for the EGDT and control groups 9.9 ± 11.1 and 11.1 ± 9.3, respectively. b For the EGDT and control groups, median (interquartile range) duration of ventilation 3 (0-7) and 4 (1-13) days and duration of vasopressor support 3 (2-5) and 3 (14-67) days, respectively. c Data presented for 10% lactate clearance group only. For the 30% lactate clearance group, duration of ventilation 4.0 ± 3.1 days. 17 Table S2. ICU admission and length of stay in ICU and hospital Author ICU admission ICU length of stay Hospital length of stay % days days EGDT Control EGDT Control EGDT Control Primary objective Rivers et al. NA NA NA NA 13.2 ± 13.8 13 ± 13.7 Jones et al. 100 100 5.6 ± 7.4 5.9 ± 8.5 12.1 ± 11.7 11.4 ± 10.9 ProCESS Investigators 91.3 85.8 5.1 ± 6.3 4.9 6.5 11.1 ± 10.0 11.8 ± 11.5 ARISE Investigators 91.5 83.0 4.9 ± 7.2 4.8 ± 5.9 14.1 ± 17.3 15.1 ± 34.2 ProMISe Investigators 88.2 74.6 5.7 ± 8.1 6.4 ± 9.8 17.6 ± 23.0 16.5 ± 24.0 Secondary objective Wang et al. 100 100 NA NA NA NA De Oliviera et al. 100 100 7 (5-18) 9 (5-17) NA NA EGDT collaborative 100 100 19.9 2.7 20.6 1.9 NA NA Tian et al.a 100 100 11.3 ± 6.0 7.9 ± 6.0a NA NA Yu et al. 100 100 10.0 ± 4.0 8.0 ± 3.0 35 ± 16 29 ± 11 Lu et al. 100 100 9.5 ± 2.5 7.1 ± 3.1 NA NA Data presented as mean ± SD or proportions (%) unless otherwise indicated. ProCESS denotes Protocolized Care for Early Septic Shock; ARISE, Australasian Resuscitation in Sepsis Evaluation; ProMISe, Protocolised Management in Sepsis; EGDT, early goal-directed therapy; NA, not available. The primary objective included only those studies in which patients presented to the emergency department with septic shock. The secondary objective included those studies in which the presenting source was the emergency department and the ward or intensive care unit or where the source was not known. The control for all analyses was usual care or another non-EGDT resuscitation strategy. For ProCESS, data presented for the control group is usual care and protocol-based standard therapy groups combined. a Data presented for 10% lactate clearance group only. ICU length of stay for 30% lactate clearance group 7.5 ± 4.0 days. 18 Table S3. Quality assessment for included studiesa Author Random sequence generation Allocation concealment Blinding of participants and personnel Blinding of outcome assessment Incomplete outcome data Selective reporting Other sources of bias Primary objective Rivers et al. Low Low High Low Low Unclear Low Jones et al. Low Low High Low Low Unclear Low ProCESS Investigators Low Low High Low Low Low High ARISE Investigators Low Low High Low Low Low Low ProMISe Investigators Low Low High Low Low Low Low Secondary objective Wang et al. Unclear Unclear High Unclear Low Unclear Unclear De Oliviera et al. Low Low High Low Low Low High EGDT collaborative Low Unclear High Unclear Low Unclear Unclear Tian et al. Low Unclear High Unclear High Unclear Unclear Yu et al. Low Unclear High Unclear Low Unclear Unclear Lu et al. Low Unclear High Unclear Low Unclear Unclear ProCESS denotes Protocolized Care for Early Septic Shock; ARISE, Australasian Resuscitation IN Sepsis Evaluation; ProMISe, Protocolised management In Sepsis; EGDT, early goal-directed therapy. The primary objective included only those studies in which patients presented to the emergency department with septic shock. The secondary objective included those studies in which the presenting source was the emergency department and the ward or intensive care unit or where the source was not known. a Quality assessment conducted independently by two assessors not involved in the design, conduct, analysis and reporting of the included studies (ADa, TI). 19 6. ACKNOWLEDGEMENTS We are grateful to Ms Sue Rockliff (Librarian, The Queen Elizabeth Hospital, Adelaide, South Australia) for assistance with the literature search. 20