Experiment #3
advertisement

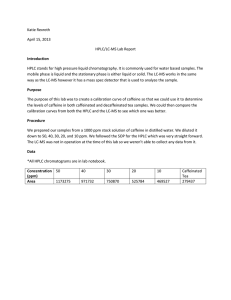
Experiment 3: HPLC and LC-MS Introduction: The instruments we will use are the HPLC and the LC-MS. The technique used for HPLC is analytical separation. HPLC separates nonvolatile species, and runs industrial substances such as amino acids, proteins, antibiotics, and other substances. The LC-MS is selective; it can isolate unresolved peaks by monitoring their masses. The LC-MS can give the details about a sample instead of relying on its retention time, like HPLC. Purpose: The purpose of the lab is to learn how to use the HPLC and the LC-MS to run standard samples of caffeine, tea samples, and an Excedrin sample. Procedure: HP-LC Make sure there is enough degassed methanol and water in flasks Turn on computer and check pump lines A and B, and make sure there are no air bubbles between flasks and HPLC. Purge the lines, by turning on pump button and detector power button. Select HPLC method icon. Choose the Taylor Test method. Set parameter according to Standard Operating Procedure and click Download button Make sure HPLC knob is switched to load Run standards and then unknowns After sample is done running, select analyze Record data, and print peaks Shut down HPLC LC-MS Make sure everything is turned on Turn on nitrogen in gas room and on the bench Click on Analyst Software icon, Hardware Configuration > LCMS > Activate Profile Click Build Acquisition Batch Fill in Set name Select method: caffeine 2011 Click on Add Set > Add Sample, select number of samples Enter numerical positions of vials in Vial Position Click Submit Click View > Sample Queue Click Ready, start sample View results and record data Deactivate LC-MS Turn off nitrogen gas on bench and in gas room Data: The graphs and data for the HPLC are attached in the notebook. The LC-MS was not working during the week that we were on these instruments, so we were not able to use that instrument. Calculations: Stock Solution: 1 𝑚𝑔 0.001 𝑔 1𝑔 100 𝑚𝑔 = = = 1𝑔 1,000,000 𝑔 100 𝑚𝐿 1000𝑚𝐿(1 𝑚𝐿) 1000 𝑔 Dilutions: M1V1=M2V2 1000(x)=(50)(50) = 1000(x)=2500 =2.5mL 1000(x)=(40)(50) = 1000(x)=2000 =2.0 mL 1000(x)=(30)(50) = 1000(x)=1500 =1.5 mL 1000(x)=(20)(50) = 1000(x)=1000 =1.0 mL 1000(x)=(10)(50) = 1000(x)=500 Caffeinated tea 413654 = 20575(𝑥) + 271686 141968 = 20575(𝑥) 6.900 = 𝑥 6.900 𝑝𝑝𝑚 Decaffeinated tea 285692 = 20575(𝑥) + 271686 14006 = 20575(𝑥) . 6807 = 𝑥 . 6807 𝑝𝑝𝑚 =0.5 mL Excedrin 509424 = 20575(𝑥) + 271686 237738 = 20575(𝑥) 11.555 = 𝑥 11.555 𝑝𝑝𝑚 Concentration vs. Area 1400000 1200000 Area (nm) 1000000 800000 y = 20575x + 271686 R² = 0.9301 600000 400000 200000 0 0 10 20 30 40 50 60 Concentration (ppm) Conclusion: The LC-MS instrument was under repair during the week of working on the liquid chromatography instruments, so we only worked on the HPLC. When first turning on the HPLC instrument we had an LK.S error, all it took was a few tries of turning the instrument off and then back on for it to work. The HPLC was an easy instrument to use the samples did not take long to run, the samples ran for about 10 minutes each. The R2 value was a little low and the 30ppm sample was not close to the line of best fit. The decaffeinated tea sample seemed to have the least ppm value.