Research Integrity and Assurance - Cornell University College of
advertisement
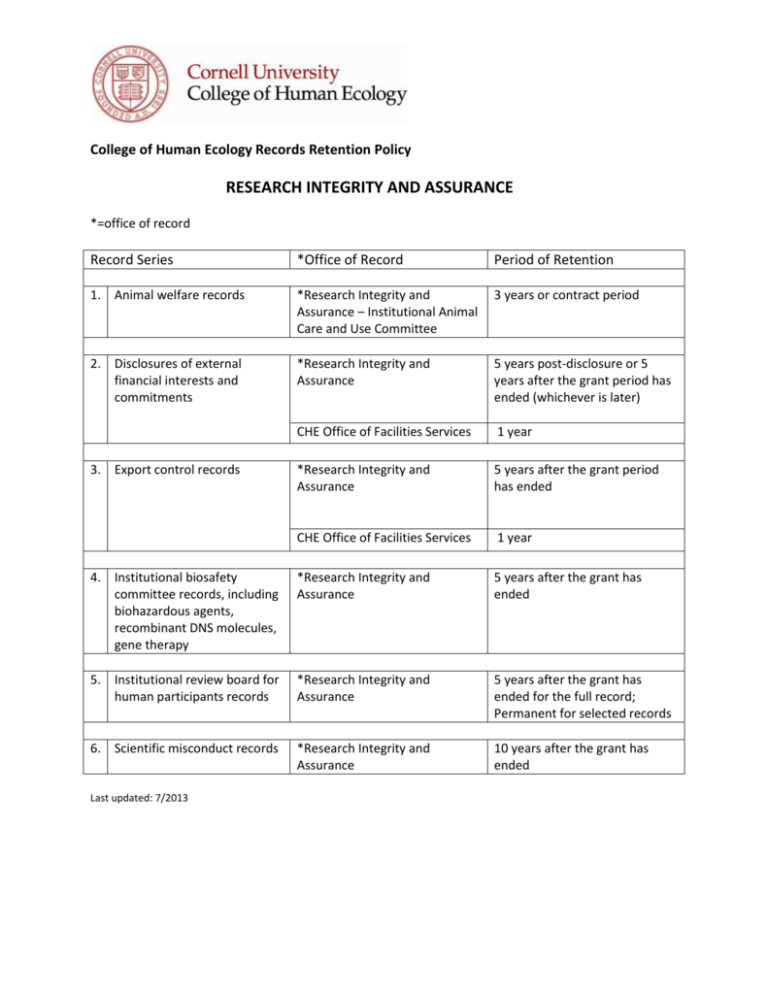
College of Human Ecology Records Retention Policy RESEARCH INTEGRITY AND ASSURANCE *=office of record Record Series *Office of Record Period of Retention 1. Animal welfare records *Research Integrity and Assurance – Institutional Animal Care and Use Committee 3 years or contract period 2. Disclosures of external financial interests and commitments *Research Integrity and Assurance 5 years post-disclosure or 5 years after the grant period has ended (whichever is later) CHE Office of Facilities Services 1 year *Research Integrity and Assurance 5 years after the grant period has ended CHE Office of Facilities Services 1 year 4. Institutional biosafety committee records, including biohazardous agents, recombinant DNS molecules, gene therapy *Research Integrity and Assurance 5 years after the grant has ended 5. Institutional review board for human participants records *Research Integrity and Assurance 5 years after the grant has ended for the full record; Permanent for selected records 6. Scientific misconduct records *Research Integrity and Assurance 10 years after the grant has ended 3. Export control records Last updated: 7/2013











