Properties of Matter: Lesson Plans
advertisement

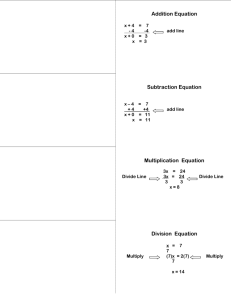
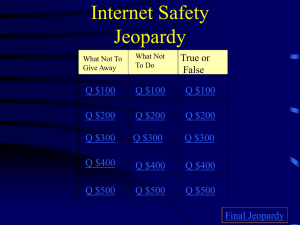
LEARNING—FOCUS STRATEGIES Lesson Planning Form Name: Sapp, Woods Class: 8th Science GPS: S8P1d: Distinguish between Date(s): October 24-Nov. 3, 2011 physical and chemical properties of matter as physical (i.e. density, melting point, boiling point) or chemical (i.e. reactivity, combustibility) S8P1c: Describe the movement of particles in solids, liquids, gases, and plasma states. Topic: Properties of Matter Plan for the Concept, Topic, or Skill—Not for the Day Learning – Focus Time Essential Question(s): (What do students need to learn to be able to answer the Essential Question?) Use K-U-D organizer to categorize/combine into 2-4 Assessment Prompts (AP) Activating Thinking Strategies: (ex: kwl, word maps, wordsplash, etc…) How do I distinguish between physical and chemical properties of matter as physical or chemical? How do I describe the movement of particles in solids, liquids, gases, and plasma states?" AP#1 Ticket out the door AP#2 Ticket out the door AP#3 Ticket out the door AP#4 Ticket out the door AP#5 Ticket out the door AP#6 Acrostic poem (density) AP#7: Acrostic poem (properties) AP#8: Jeopardy GameAP#9:Test Preview Vocabulary Words: Physical property mass volume strength elasticity brittleness ductility solid liquid gas density chemical property flammability reactivity characteristic properties Display 3 items in a beaker (baking soda, water, and a rock). Ask students to write a description of each substance. Discuss then explain that their descriptions include physical characteristics of substances. Ask students if they ever seen sour milk. Have the students to write a description about sour milk. Explain to students that sour milk is a result of a chemical reaction that occurs in fresh milk. Tell students properties of fresh milk are different then properties of sour milk. Why do we research information? Which is heavier on pound of feathers or one pound of bricks? List some words on the board have students classify as chemical or physical property. (color, flammability, combustion, shape, state of matter, reactivity) Discuss the 3-2-1 from Friday. What is the difference between chemical and physical properties? Teaching Strategies: AP’s are your embedded Summarizers throughout instructional period Graphic Organizers: Foldable Instruction for AP#1: Watch safari montage : Properties of Matter Then read pg. 298-299 Summary point 1-What is matter? Summary point 2-Write a summary about video. Summary point 3: What two factors does density depend? AP#1 Name 2 physical properties. States of matter Chemical and physical properties Double Bubble Map Chemical and Physical Properties Cornell Note-Taking Format Word splash Frayer Model for vocabulary words Instruction for AP#2: Students will match questions with answers for notes instead of taking traditional notes. They will work with partners. Then will review notes to clarify any confusion Summary Point 1-Write a paragraph explaining what you learned from this activity. AP#2:List 2 chemical properties. Instruction for AP#3 Students will be in computer lab to do background research for science fair project or research paper on energy. Summary point 1-How do you know your resource is valid? AP#3:List 5 resources for your paper. Instruction for AP#4: Create a foldable for states of matter Graphing activity with mass and volume. Read pg 300-302 Do 5 guided practice density problems. Answer review questions pg 302 Summary point 1-Which state of matter moves by vibrations? Summary point 2-Which variable is labeled on the y-axis? Ap#4: What does the slope tell you about this graph? Instruction for AP#5 Complete guided reading for chapter 12 Summary point 1-Compare/Contrast chemical and physical properties AP#5: 3-Things I learned 2-Interesting facts I discovered 1-Questions I still have Instruction for AP#6: Graphing mass/volume practice calculating density; density lab using density cubes Summary point 1: What is density? Summary point 2: What is the formula for calculating density? Summary point 3: How do you calculate the volume of an irregular shape object? Summary point 4: How do you calculate the volume of a regular shape object? (box) AP#6: Density-Summarize the concept by acrostic poem (density) Instruction for AP#7: Create a foldable for chemical and physical properties which will look like a chart. Name whether it is a chemical or physical property, definition, example. Then complete handouts physical or chemical and crossword to help review vocabulary. Quiz on vocabulary words Summary point 1: Name 2 examples of chemical properties Summary point2:Name 4 states of matter. AP#7: Properties acrostic poem to summarize Assignment and/or Assessment: Instruction for AP#8: Review for test by doing Jeopardy or board game Summary point1: Write one thing you may still be confused about. Summary point 2: Write two multiple choice questions that you want to see on your test. AP#8: Jeopardy Game Instruction for AP#9: Test on Properties of matter AP#9: Test Unit Organizer Section review questions 12.1 pg302 (CPO Textbook) Quiz Review for Test using board game or Jeopardy Final Assessment-Unit Test Re-Teaching Focus and Strategy: (if necessary) SED Modifications: Materials and Resources Reinforcement handout: Properties of matter; chemical and physical properties Foldable Chart Table for states of matter and properties of matter Crossword This review vocabulary worksheet DH,CC, JP, EJ, DM, WE,-read aloud, repetition, paraphrase directions, extended time JA, MA-small groups( send to media center to test) ESOL Collaborative teacher-AV, AV, GP JH,PP, IG,-read aloud, repetition, paraphrase directions extended time CPO Physical Science Textbook Unit Organizer Science Holt chapter 2 Board Games and Unit Test