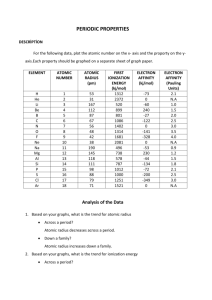
File

Chapter 6 WS SI
CHM 111 Summer 2013
SI Leader: Kristen Kelly
Key Terms:
Electron affinity negative electron affinity ion electron configuration ionization energy second ionization energy octet rule cation anion
Zeff atomic radii lattice energy ionic bond ionic solid
1.
Metals tend to __________ e forming ___________ while non-metals tend to ________e forming _________.
2.
How many protons and electrons are in the following ions? a.
Rb + b.
Se 2-
3.
Element X 2+ has 36 electrons and _____ protons and is ________.
4.
Write the electron configuration for the following ions: a.
Na + b.
O 2c.
Fe 2+
5.
Explain why the first ionization energy of carbon is less than the first ionization energy of nitrogen but the electron affinity of carbon is greater than the electron affinity of nitrogen?
6.
Circle the correct answer in bold: Generally cations are smaller/larger than their corresponding atoms and anions are smaller/ larger than their corresponding atoms.
7.
State if the following trends increase or decrease going from left to right and top to bottom on the PT. a.
electron affinity b.
ionization energy c.
atomic radius d.
Zeff
8.
Circle the atom as described a.
highest electron affinity C N B b.
highest ionization energy Ne Na N c.
lowest ionization energy Ne Na N d.
largest atomic radius S 2 Cl Cl e.
largest first ionization energy Se S Te
Chapter 6 WS SI
CHM 111 Summer 2013
SI Leader: Kristen Kelly f.
highest second ionization energy Mg Na Al g.
more negative electron affinity Mg Mg 2+ Br
9.
Calculate the overall change in energy in kJ/mole for the formation of CsF (s) from its elements using the following data:
Ea for F (g) = -328 kJ/mol
Ei1 for Cs (g) = +375.5 kJ/mol
Ei2 for Cs (g) = +2422 kJ/mol
Heat of sublimation for Cs (s) = 76.1 kJ/mol
Bond dissociation energy for F
2
= 158 kJ/ mole
Lattice energy for CsF (s) = 740 kJ/mol
10.
Arrange the following cations from largest to smallest lattice energy when combined with F . a.
Na, K, Li
11.
Arrange the following anions from largest to smallest lattice energy when combined with Na + . a.
I, Br, Cl
12.
Order the following compound from largest to smallest lattice energy. a.
MgCl
2
, AlCl
3
, NaCl
13.
For the following reaction predict the product. If no reaction occurs, then write no reaction. a.
Sr + O
2
→ b.
Ca + H
2
O → c.
Li + NH
3
→ d.
Ca + X
2
→ e.
Mg + Ne → f.
F
2
+ H
2
→