Atomic Structure Graphic Organizer
advertisement

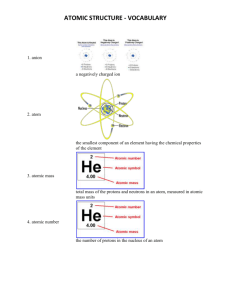
Atomic Structure SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of proton, electron, and neutron locations. atomic mass and atomic number. atoms with different numbers of neutrons (isotopes). explain the relationship of the proton number to the element’s identity. Name of Subatomic Particle Characteristics of the Subatomic Particles Location of Mass of Subatomic Subatomic Particle Particle Neutron Proton Electron Carbon Atom Charge of Subatomic Particle How to Read a Periodic Table Tile 4 Be Beryllium 9.01 How to Find the Number of Subatomic Particles for an Atom Subatomic Particle Neutron Proton Electron How to find the number of Atomic Structure SPS1. Students will investigate our current understanding of the atom. a. Examine the structure of the atom in terms of proton, electron, and neutron locations. atomic mass and atomic number. atoms with different numbers of neutrons (isotopes). explain the relationship of the proton number to the element’s identity. Name of Subatomic Particle Characteristics of the Subatomic Particles Location of Mass of Subatomic Subatomic Particle Particle Charge of Subatomic Particle Neutron nucleus 1 AMU Neutral Proton nucleus 1 AMU +1 Electron Electron cloud In energy levels 0 -1 Carbon Atom How to Read a Periodic Table Tile 4 Be Beryllium 9.01 Atomic Number = the number of protons and the identity of the atom Element Symbol Element Name Atomic Mass = the total number of neutrons and protons How to Find the Number of Subatomic Particles for an Atom Subatomic Particle How to find the number of Neutrons Atomic Mass rounded to the nearest whole number minus the Atomic Number Protons Equal to the Atomic Number Electron Equal to the number of protons