Subatomic Particles and Elements Notes
advertisement

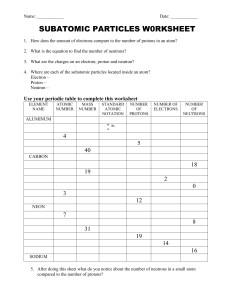
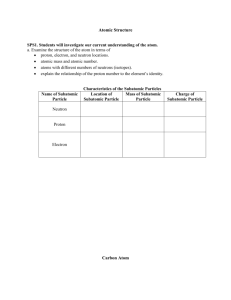
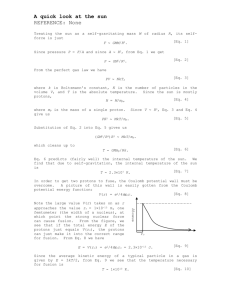
Chapter 4: Atoms and Elements Date:____________ Section 3: Subatomic Particles and Element - Notes Objectives: Describe the respective properties and charges of electrons, neutrons, and protons. Determine the atomic symbol and atomic number for an element using the periodic table. Protons: Proton: a ______________________________________________ subatomic particle (p) o Located in the __________________________ o 1 proton has a charge of ________ o Actual Mass = ________________________________ o Relative Mass = ________________________________ Elements are defined by their numbers of _______________________. o If an atom had a __________________________ number of protons, it would be a __________________________ element. The number of protons in the nucleus of an atom is its __________________________________ and is given the symbol ______. o Hydrogen always has ______ proton o Carbon always has ______ protons How many protons are in: o o Argon? Plutonium? Electrons: Electron: a ______________________________________________ subatomic particle (e-) o Located in the _____________________________________________ the nucleus o 1 electron has a charge of _______ o Actual Mass = ___________________________ o Relative Mass = ___________________________ About _______________ of the mass of a proton (small enough to be negligible) In a neutral atom, the number of electrons is ______________________ the number of protons. o Hydrogen has _____ electron o Carbon has _____ electrons How many electrons are in? o Argon? o Plutonium? Neutrons: Neutron: a subatomic particle that has ________________________ (n) o Located in the __________________________ o Actual Mass = ______________________________ o Relative Mass = _______________________________ The number of neutrons in an atom can _______________. Electrical Charge: ________________________________________ is a fundamental property of protons and electrons. o Positive and negative electrical charges _________________ each other. o Positive–positive and negative–negative charges _________________ each other. o Positive and negative charges ________________ each other so that a proton and an electron, when paired, are charge-neutral. Matter normally has a ________________________________________ o equal numbers of positive and negative charges that _____________________________ In an electrical storm, the charge balance of matter is __________________________________. The quick rebalancing of charge often occurs in dramatic ways, such as is seen in ________________________. Elements: It is the number of ___________________________ in the nucleus of an atom that identifies the atom as a particular _______________________________. The periodic table of the elements lists all known elements according to their _____________________________. Most chemical symbols are based on the _______________________________ of the element. o Hydrogen (_____), Carbon (_____), Oxygen (_____) Some symbols are based on ________________________________________. o Potassium (_____) – from the Latin ________________ o Sodium (_____) – from the Latin ________________ Additional elements with symbols based on their ___________________________________ names include the following: o lead (_____), mercury (_____), iron (_____), silver (_____), tin (_____), copper (______) Early scientists gave newly discovered elements names that reflected their _________________ Other elements were named after _______________________ Other elements were named after ______________________. List the atomic symbol and atomic number for each element. o Silicon o Potassium o Gold o Antimony