Atoms Enrichment Activities: Quarks, Accelerators, Models
advertisement

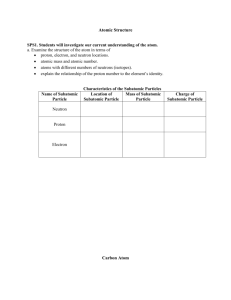
Enrichment Opportunities: Atoms Read the introductory information and then choose ONE of the enrichment activities listed below. How Low Can They Go? The never-ending quest to better understand our universe has led scientists to some amazing places, from the deep reaches of outer space to the heart of the atom. Our curiosity has shown us things smaller than anyone thought existed – first the atom and then subatomic particles. But scientists didn’t stop with protons, electrons, and neutrons. Instead, they devised sophisticated instruments called particle accelerators to take a close look at subatomic particles. What they found were even smaller subatomic particles, which they named quarks, positrons, and gluons. Will they keep looking for something even smaller? Of course! The Quirks of Quarks Find out more about subatomic particles like quarks. How many different kinds of quarks are there? Where do quarks fit into the atomic model? Do they carry a charge? What is their mass? Write a scientific magazine article about the subatomic particle you research. Particle Accelerators and Cyclotrons How do you dig into a nucleus? Find out about particle accelerators and cyclotrons in your library and on the Internet. How do they work? How many particle accelerators or cyclotrons exist in the United States? in the world? When was the first particle accelerator built? How big are they? Write a newspaper article to report your findings. Copyright by Holt, Rinehart and Winston Model of the Atom Purpose: To construct a visual representation of an atom. Procedure: 1. 2. 3. 4. 5. Select an element. Represent each subatomic particle with beads, pom poms, clay, etc. Represent each type of subatomic particle with a different color. Make the electrons smaller than the protons and neutrons. Make the protons and neutrons the same size. Attach a color key to the project. Include the symbol, atomic number, and number of protons, neutrons and electrons in your atom, and your name and homeroom on the color key.