KrakhalevaNV_eng
advertisement

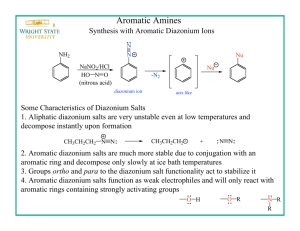
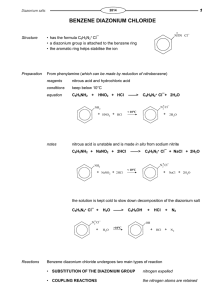
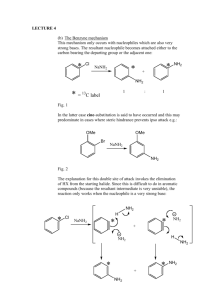
Research of behavior of aryldiazonium tosylates in electrophilic substitution reactions with the formation of new C-N bonds on the example of the reaction to produce azomethines. N.V. Krakhaleva, P.V. Petunin, M.E. Trusova, P.C. Postnikov TPU, 634050, Russia, Tomsk, Lenina, 30 E-mail: kraxalevanv@tpu.ru Electrophilic substitution reactions CH-acids with aromatic diazonium salts induce of great interest for obtaning Schiff's bases. For today, we know different reaction CH-acids with aromatic diazonium salts, when they used as N-electrophile [1,2]. All of them have different disadvantages, such as low yield products. Moreover, tetrafluoroborates and chlorides phenyldiazonium have highly fire and explosion hazard. A new type of aromatic diazonium salts – aryldiazonium tosylate (ADT) was synthesized scientific group Filimonov V.D. [3]. The authors indicate their high stability. This type of salts are explosion-proof and shows high reactivity in many transformations. We have carried out research where ADT show itself as N-electrophile by reaction with CHacid. As we can see, product yields are almost nearly quantitative and do not depend on the nature of substituents. The interaction of passed under the general scheme: Thus, we showed that ADT exhibit high reactivity as N-electrophiles and selectivity in reactions with CH-acids. Literature 1. F. Abdel-Wahab and F. Salwa, Monatsh. Chem., 2008, 139, 1083-1090. 2. M. Alafeefy, S. Isik and D. Vullo, Bioorg. & Med. Chem., 2013, 21, 1396-1403. 3. V. Filimonov, M. Trusova, P. Postnikov and E. Krasnokutskaya, Org. Lett., 2008, 10(18), 3961-3964.