Lab Report 2
advertisement

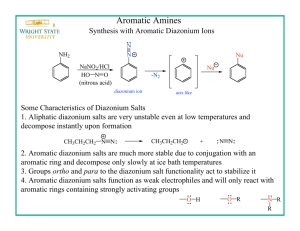
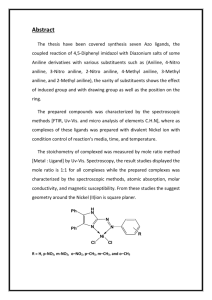
Thomas Mackay tbm458 CH 128L – Majors Discussion: The purpose of this lab is to synthesize azo violet via multistep synthesis. Today dye is synthetically made; the most common dyes are called azo dyes, which are formed from aromatic amines to phenols and other aromatic amines. They are called azo dyes due to their nitrogen-nitrogen double bond, which is known as azo group. Azo dyes are stabilized by extended conjugation. The first step of the reaction involves formation of the diazonium ion from the aromatic amine by adding nitrous acid in a process known as diazotization. After this step, the phenol or amine is added in a reaction known as diazonium coupling. The synthesis involves a total of five steps, starting with aniline – a phenyl group attached to an amino group. Aniline is a weak base. Aromatic anilines, such as aniline, are generally much weaker bases than aliphatic amines because of the electron-withdrawing effect of the phenyl group. Thus, aniline reacts with strong acids to form the anilinium ion, which is also known as phenylammonium. The weak basicity is due to two factors: an inductive effect from the more electronegative sp2 carbon and a resonance effect because the lone pair on the nitrogen is partially delocalized into the pi system of the benzene ring. The pKa of the conjugate acid of aniline is 4.63. The formation of acetanilide is an example of a protection group and is done to make the formation of p-nitroaniline much easier. By acetylating aniline, the reactivity of the amine is decreased. Amines are strong ortho-para directors. If left unprotected, then the result would be a mixture of the ortho and para product as well as some multi-nitrated product. The acetyl group prevents these undesired outcomes by being too bulky, causing steric hindrance and not allowing for the formation of the ortho product, although there will be trace amounts of it. In addition, the acetyl group ties up the lone pair of electrons in the amine through resonance, making the dinitration products less probable. Therefore, the predominant product is the p-nitroacetanilide, requiring a simple step to remove the small amount of ortho product that is made. Following the synthesis of p-nitroacetanilide, deprotection via acid workup produces pnitroaniline. At this point, synthesis of azo violet can be carried out. The first step is to perform the diazotization product of p-nitroaniline. The next step is to run the diazonium coupling reaction with resorcinol, also known as 1,3-dihydroxybenzene. The reaction of a primary amine with nitrous acid produces the diazonium ion. This conversion of a primary amine to a diazonium ion is known as diazotization, and the diazonium ion is given by ArN2+ or RN2+. With a small amount of cleanup, the final product is azo violet. Azo violet, of course, is a dye, so the next step is to see if it can dye some fabric. It can also be used as a pH indicator, so it will then be tested for different colors in different pH values. Electrophilic aromatic substitution is a reaction whereby an electrophile, E+, substitutes for a hydrogen that resides on an aromatic ring. There is a general two-step mechanism for electrophilic aromatic substitution: The first step involves the attack of the strong electrophile, E+, by the weakly nucleophilic pi electrons of the aromatic ring, which produces a resonance-stabilized cation intermediate; this step is rate-determining. The second step involves the loss of H+ from the previously generated cation intermediate, which regenerates aromaticity in the ring and yields the product; this step is faster than the first. There are three fundamental kinds of substituents that can be found on an aromatic molecule. The first is an electron-releasing group, which is activating and, therefore, always ortho-para directing. In addition, this group increases the rate of substitution and increases the nucleophilicity of the ring. The second is an electron-withdrawing group – other than the halogens – which is deactivating and, therefore, meta directing. In addition, this group decreases the rate of substitution and decreases the nucleophilicity of the ring. The third is a halogen, which demonstrates deactivation but is ortho-para directing. In this case, the ratio of the amounts ortho to para products is affected by electronegativity and steric hindrance. A resonance effect that delocalizes the charge of the cation intermediate will lower the activation energy associated with its formation and will therefore have an activating effect toward further electrophilic aromatic substitution. Some example substituents that exhibit this resonance effect are –NH2, –OR, and –OH. A resonance or inductive effect that decreases electron density on the ring will deactivate the ring to further substitution. Some example substituents that exhibit either of these effects include – NO2, –SO2 –, –SO3H, and –C=O. Conclusion: IR Spectroscopy: Aromatic rings have a medium to weak peak in the C-H stretching region around 3030 cm-1, evidence of (sp2-1s) =C-H bonds. Aromatic rings also show strong absorption in the region between 690 and 900 cm-1, evidence of out-of-plane C-H bending. Further, aromatic rings show many absorptions between 1450 and 1600 cm-1, evidence of C=C stretching. Infrared absorptions of both primary and secondary amines are caused by N–H stretching vibrations observed in the range between 3300 and 3500 cm-1. Similar to O–H bonds, N–H bonds tend to be broader and shift to longer wavelength when taking part in hydrogen bonding. For amides, the carbonyl double bond stretching vibration is found between 1630 and 1680 cm-1 with strong intensity. For a secondary amide, the single N–H stretching vibration is found between 3200 and 3400 cm-1 with medium intensity. For the nitro group, the N–O asymmetric stretch is found between 1550 and 1475 cm-1, whereas the N–O symmetric stretch is found between 1360 and 1290 cm-1. Melting Point: The actual melting point range of acetanilide is 113-115 °C. The actual melting point range of p-nitroacetanilide is 213-215 °C. The actual melting point range of p-nitroaniline is 146149 °C. In each case, the experimental melting point range was slightly below the actual melting point range. This is the result of small amounts of water and contamination. Because the experimental melting points were so similar to their actual melting points, the identities of the three different molecules were verified adequately. Dyeing Test: After carrying out the procedure for this test, the cotton remained more or less its original color with a hint of orange. The cotton turned violet when soaked with a few drops of 0.5 M NaOH, however. Indicator Test: When a few crystals of the dye were placed into three different test tubes, nothing happened in both the test tube that contained 0.5 M HCl and the test tube that contained a few drops of water. However, the dye changed from orange to violet in the test tube that contained 0.5 M NaOH. Percent Yield: The calculations for percent yield can be found in the Data and Results. o o o Acetanilide: The mass obtained was 2.402 grams, which amounted to a percent yield of 45.03%. P-nitroacetanilide: The mass obtained was 0.535 grams, which amounted to a percent yield of 40.14%. P-nitroaniline: The mass obtained was 0.369 grams, which amounted to a percent yield of 65.08%. These percentages are quite close to the expected ranges typically given by Fjetland. However, loss of product can be attributed to improper lab technique, imperfect removal of product from the glassware, and vacuum filtration stealing some product through the holes.