Ex 4 Azo Dyes
advertisement
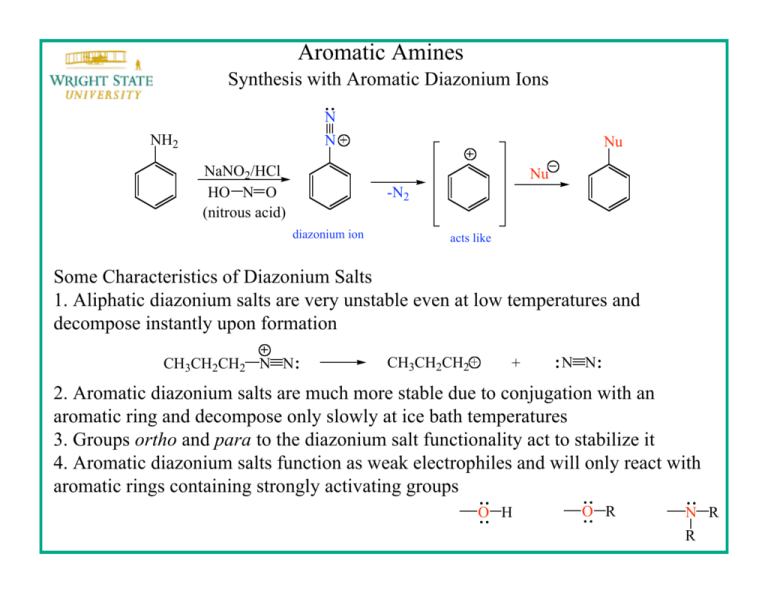
Aromatic Amines Synthesis with Aromatic Diazonium Ions N NH2 N NaNO2/HCl HO N O (nitrous acid) Nu Nu -N2 diazonium ion acts like Some Characteristics of Diazonium Salts 1. Aliphatic diazonium salts are very unstable even at low temperatures and decompose instantly upon formation CH3CH2CH2 N N CH3CH2CH2 + N N 2. Aromatic diazonium salts are much more stable due to conjugation with an aromatic ring and decompose only slowly at ice bath temperatures 3. Groups ortho and para to the diazonium salt functionality act to stabilize it 4. Aromatic diazonium salts function as weak electrophiles and will only react with aromatic rings containing strongly activating groups O H O R N R R Azo Dyes Aromatic diazonium ions can be trapped (before loss of N2) by electron-rich aromatic compounds (e.g., possessing activating groups) O2N NH2 NaNO2/HCl 0 oC Cl O2N an aromatic diazonium salt N N 4-Nitrobenzenediazonium chloride 4-Nitroaniline NO2 Cl O2N N N OH + N 2-Naphthol Para Red N OH Azo Dyes Two azo dyes are prepared: para red and one from another naphthol-aromativ amine combination Sodium Nitrite Solution: dissolve NaNO2 (1 g) in H2O (10 mL). This provides enough 10% NaNO2 solution to prepare two azo dyes Aromatic Amine Solution: disolve aromatic amine (250 mg) in 10% HCl (10 mL) Naphthol Solution: dissolve naphthol (250 mg) in 10% NaOH (10 mL) Diazotized Amine Solution: cool the aromatic amine solution to 0 oC and add sodium nitrite solution (5 mL) dropwise with mixing. Allow the solution to stand 2 min Azo Dye Solution: insert a cloth strip into diazotized amine solution, allow to become saturated and add the naphthol solution dropwise Azo Dyes This procedure is to be used employing 4-nitroaniline and 2-naphthol to prepare para red and a second azo dye using a different combination of amine and naphthol Aromatic Amines Naphthols OH O2N NH2 4-Nitroaniline 2-Naphthol OH NH2 NO2 2-Nitroaniline 1-Naphthol Rinse the cloth samples with water to determine color-fastness Synthesis with Aromatic Diazonium Ions What starting materials are needed to synthesize the following azo compound? O O S Na O N N methyl orange O Na O S O N N Cl + Me N Me Me N Me