BIOLOGY POWERPOINT Midterm Review CHAPTER 1 Introduction
advertisement

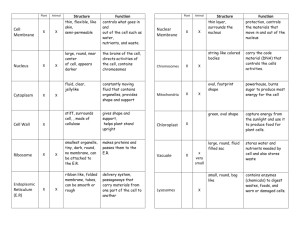
BIOLOGY POWERPOINT Midterm Review CHAPTER 1 Introduction to Biology THE STEPS OF THE SCIENTIFIC METHOD 1. MAKE AN OBSERVATION 2. FORM A HYPOTHESIS a) A PREDICTION(EDUCATED GUESS?) 3. TEST THE HYPOTHESIS WITH AN EXPERIMENT I. EXPERIMENTS SHOULD HAVE ONLY 2 VARIABLE II. INDEPENDENT VARIABLE –the thing that YOU change III. DEPENDENT VARIABLE- the thing that changes in response IV. A CONTROL IS SOMETHING HELD CONSISTENT FOR ALL 4. COLLECT AND ANALYZE DATA a) WE USE MATHEMATICAL-QUANTITATIVE DATA I. GRAPHS, TABLES AND MEASURES LIKE AVERAGES, MEDIANS.. 5. FORM A CONCLUSION I. DOES THE DATA SUPPPORT YOUR HYPOTHESIS 6. SHARE YOUR DATA* I. EXPERIMENTAL DATA SHOULD BE REPLICABLE 3 LEVELS OF SCIENTIFIC CERTAINTY 1. A HYPOTHESIS IS THE LOWEST LEVEL 1. A PREDICTION TO EXPLAIN YOUR OBSERVATION (EDUCATED GUESS?) 2. A THEORY IS A CONSENSUS WITHIN THE SCIENTIFIC COMMUNITY a. A GENERAL EXPLANATION FOR A BROAD RANGE OF DATA b. USES DATA FROM A VARIETY OF SOURCES FOR SUPPORT c. EXAMPLE –THE EXTINCTION OF DINOSAURS 3. A LAW HAS NO SIGNIFICANT DATA TO OPPOSE IT a. DOES THE DATA SUPPPORT YOUR HYPOTHESIS b. EXAMPLE – E = mc2 THE BRACHES OF BIOLOGY –BIOLOGY IS THE THE STUDY OF ORGANISMS FOR EACH BRANCH OF BIOLOGY WE STUDY HOW ORGANISMS.. 1. ECOLOGY – INTERRACT WITH THE ENVIRONMENT 2. CELL BIOLOGY- CELLS AND THEIR STRUCTURES 3. GENETICS – HOW TRAITS ARE INHERITED OR HEREDITY 4. BIOCHEMISTRY- CHEMISTRYOF LIFE OR METABOLIC PROCESSES 5. EVOLUTIONARY BIOLOGY –HOW LIFE EVOLVES 6. MICROBIOLOGY – MICROSCOPIC ORGANISMS 7. BOTANY - PLANTS 8. ZOOLOGY – ANIMALS 9. PHYSIOLOGY – HUMAN BODY THE 7 CHARACTERISTICS OF LIFE AN ORGANISM: IS A LIVING THING CAPABLE OF CARRYING ON ALL THE PROCESS OF LIFE ALL ORGANISMS SHARE THE FOLLOWING TRAITS… 1. CELLULAR ORGANIZATION 5. REPRODUCTION 2. HOMEOSTASIS 6. GROWTH AND DEVELOPMENT 3. HEREDITY 7. METABOLISM 4. RESPONSIVENESS THE 7 CHARACTERISTICS OF LIFE: ALL ORGANISMS SHARE THE FOLLOWING TRAITS… 1. CELLULAR ORGANIZATION a) ORGANISMS ARE EITHER UNICELLULAR OF MULTICELLULAR I. UNICELLULAR -1 CELLED II. MULTICELLULAR – MORE THAN 1 CELL b) THEY ARE PROKARYOTIC OR EUKARYOTIC I. PROKARYOTIC –LACK A NUCLEUS OR ORGANELLES II. EUKARYOTIC – HAVE NULCEUS AND ORGANELLES 2. HOMEOSTASIS 1. THE ABILITY TO MAINTAIN A STABLE INTERNAL ENVIRONMENT 2. EXAMPLE –THERMOREGULATION, BLOOD PRESSURE, BLOOD GLUCOSE….. 3. HEREDITY 1. ORGANISMS PASS THEIR TRAITS TO THEIR OFFSPRING 1. THEY USE THE MOLECULE DNA ORGANIZED IN CHROMOSOMES 2. THESE INHERITED TRAITS CHANGE OVER TIME – EVOLVE 1. SPECIES TRAITS ARE ENCODED IN DNA WHICH CHANGES 4. RESPONSIVENESS 1. ORGANISMS RESPOND TO THE ENVIRONMENT 1. INDIVIDUALS RESPOND WITH BEHAVIORS 2. SPECIES RESPOND BY EVOLVING 5. REPRODUCTION a. SEXUAL REPRODUCTION 1) THE EXCHANGE AND COMBINATION OF DNA 2) SEX CELLS (GAMETES)COMBINE TO FORM NEW INDIVIDUALS b. 3) HIGH LEVEL OF VARIATIONS ASEXUAL REPRODUCTION 1) THE REPLICATION OF PARENTS TO FORM DAUGHTER CELL 2) OFFSPRING IDENTICAL TO PARENTS 3) EXAMPLE –BINARY FISSION 6. GROWTH AND DEVELOPMENT a) ORGANISM GROW BY MITOSIS b) ORGANISMS DEVELOP THROUGH CELLULAR DIFFERENTIATION 7. METABOLISM 1. 2. THE SUM OF ALL CHEMICAL REACTIONS IN LIVING SYSTEMS THE CARBON CYCLE IS THE a) PHOTOSYNTHESIS I. CO2 + H2O --- C6H12O6 + O2 b) CELL RESPIRATION I. C6H12O6 + O2 --- CO2 + H2O FUNDAMENTAL PROCESS CHAPTER 18 CLASSIFICATION THE CLASSIFICATION OF ORGANISMS 1. TAXONOMY -THE SCIENCE OF CLASSIFYING ORGANISMS a) CARL LINNAEUS-THE FATHER OF MODERN TAXONOMY b) BINOMIAL NOMENCLATURE –SCIENTIFIC NAME I. A 2 NAME SYSTEM II. Genus species or Genus species III. SCIENTIFIC NAMES ARE GIVEN IN LATIN c) CLADISTICS IS THE ORGANIZATION OF ORGANISMS ON THE BASIS OF SHARED TRAITS I. WE CAN USE A CLADOGRAM TO LINK RELATED ORGANISMS 1. TAXONOMY -THE 3 DOMAIN SYSTEM a) ORGANISMS ARE IDENTIFIED BY CHARACTERISTICS I. ARCHAEBACTERIA- ANCIENT BACTERIA-EXTREMOPHILES II. BACTERIA- STREPTOCOCCUS, STAPHYLOCOCCOUS III. EUKARYA- PROTISTA, FUNGI, PLANTAE, ANIMALIA THE CLASSIFICATION OF ORGANISMS 1. 7 LEVELS OF THE MODERN TAXONOMY a) DOMAIN b) KINGDOM c) PHYLUM d) CLASS e) ORDER f) FAMILY g) GENUS h) SPECIES i) 1. THE HUMAN CLASSIFICATION a) DOMAIN – EUKARYA b) KINGDOM - ANIMALIA c) PHYLUM - CHORDATA d) CLASS - MAMMALIA e) ORDER - PRIMATE f) FAMILY - HOMINIDAE g) GENUS - HOMO h) SPECIES - SAPIENS CHAPTER 3 CHEMISTRY OF LIFE THE CHEMISTRY OF LIFE 1. MATTER-IS COMPOSED OF ATOMS a) ATOMS ARE COMPOSED OF SUBATOMIC PARTICLES I. PROTON a) FOUND IN THE NUCLEUS b) POSITVE CHARGE +1 c) MASS IS 1 ATOMIC MASS UNIT (AMU) II. NEUTRON a) FOUND IN THE NUCLEUS b) NEUTRAL CHARGE c) MASS IS 1.01 ATOMIC MASS UNIT (AMU) III. ELECTRON a) FOUND IN THE ORBITAL ENERGY SHELLS b) NEGATIVE CHARGE -1 c) MASS IS 0.01 ATOMIC MASS UNIT (AMU) THE CHEMISTRY OF LIFE 1. AN ELEMENT IS A TYPE OF ATOM a) AN ELEMENT IS SUBSTANCE MADE UP OF THE SAME TYPE OF ATOMS I. ELEMENTS OF THE SAME SUBSTANCE HAVE THE SAME NUMBER OF PROTONS GIVEN BY THE ATOMIC NUMBER a) CARBON 6 b) OXYGEN 8 c) HYDROGEN 1 II. SOME ATOMES OF THE SAME SUBSTANCE MAY HAVE DIFFERENT NUMBERS OF NEUTRON a) CARBON12 -6 PROTONS 6 NEUTRON b) CARBON14 -6 PROTONS 8 NEUTRON c) THEY ARE CALLED ISOTOPES OF CARBON THE CHEMISTRY OF LIFE THE PERIODIC TABLE ORGANIZES ALL ATOMS ITS CONTAINS THE FOLLOWING FOR EACH ELEMENT a) CHEMICAL SYMBOL I. AN ABBREVIATION OF AN ATOMS NAME a) C-CARBON, H-HYDROGEN b) SOME ARE UNUSUAL Na- Sodium, K-Potassium b) ATOMIC NUMBER I. AN ATOMS PROTON NUMBER a) CARBON HAS 6 PROTONS –ATOMIC NUMBER 6 b) HYDROGEN HAS 1 PROTON – ATOMIC NUMBER 1 c) ATOMIC MASS I. THE COMBINATION OF THE MASS OF PROTONS AND NEUTRONS a) CARBON – 6 PROTONS + 6 NEUTRON = ATOMIC MASS 12 b) SOME ARE UNUSUAL Na- Sodium, K-Potassium THE CHEMISTRY OF LIFE 1. CHEMICAL BONDS – ATOMS COMBINE THERE ARE 3 IMPORTANT BONDS a) COVALENT BOND I. BASED ON THE SHARING OF ELECTRONS a) FORM MOLECULES b) DRIVEN BY THE OCTET RULE- WHICH STATES “MOST ATOMS REQUIRE 8 ELECTRONS IN THE OUTERMOST ORBITAL SHELL-(VALENCE SHELL) c) EXAMPLES INCLUDE CO2 , C6H12O6, O2 b) IONIC BONDS I. BASED ON THE DONATION OR ACCEPTANCE OF ELECTRONS a) FORM IONIC COMPOUNDS b) ATTRACTIONS BETWEEN CHARGED ATOMS CALLED IONS c) DRIVEN BY THE ELECTRONEGATIVITY OF AN ELEMENT d) EXAMPLES INCLUDE NaCl, NaOH, HCl c) HYDROGEN BONDS I. BASED ON ATTRACTIONS BETWEEN H+ ATOMS AND OXYGEN a) IMPORTANT IN WATER, DNA , & PROTEIN STRUCTURE THE CHEMISTRY OF LIFE WATER - LIFE IS DEPENDENT UPON THE CHARACTERISTICS OF WATER a) WATER IS A POLAR MOLECULE I. IT IS A MOLECULE WITH IONIC CHARACTER a) OXYGEN IN WATER CARRIES A PARTIAL (-) CHARGE b) HYDROGENS IN WATER CARRIES A PARTIAL (+) CHARGE c) ADJACENT MOLECULES ARE ATTRACTED TO ONE ANOTHER b) LIQUID WATER IS LESS DENSE THAN SOLID WATER I. ICE FLOATS a) FLOATING ICE PREVENTS LAKES AND OCEANS FROM FREEZING COMPLETELY AND ALLOWS ORGANISMS TO LIVE IN COLD CONDITIONS b) ATTRACTIONS BETWEEN CHARGED ATOMS CALLED IONS c) DRIVEN BY THE ELECTRONEGATIVITY OF AN ELEMENT d) EXAMPLES INCLUDE NaCl, NaOH, HCl c) WATER IS STICKY I. IT IS BOTH ADHESIVE AND COHESIVE a) COHESION WATER STICKS TO ITS SELF b) ADHESIONWATER STICKS TO OTHER POLAR MOLECULES d) WATER IS THE UNIVERSAL SOLVENT I. IT DISSOLVES POLAR MOLECULES WATER –THE UNIVERSAL SOLVENT a) A SOLUTION CONTAINS BOTH A SOLVENT AND SOLUTE I. A SOLUTE IS THE SOLID DISSOLVED IN SOLUTION II. THE SOLVENT IS THE LIQUID COMPONENT OF A SOLUTION a) POLAR SOLVENTS DISSOLVE POLAR COMPOUNDS a) EXAMPLE WATER AND SUGAR b) NON-POLAR SOLVENTS DISSOLVE NON-POLAR COMPOUNDS a) EXAMPLE GASOLINE AND OIL c) SOLUTIONS WHERE WATER IS THE SOLVENT ARE CALLED AQUAEOUS a) ACIDS AND BASES ARE AQUAEOUS SOLUTIONS b) THE pH scale measures H+ ion concentration I. ACIDS –LOW pH a) H+ DONORS b) EXAMPLES INCLUDE LEMON JUICE, HCl II. BASES - HIGH pH a) H+ ACCEPTORS b) EXAMPLES INCLUDE, NaOH, OVEN CLEANER, LYE, AMMONIA BUFFERS – IONIC COMPOUNDS IN LIVING SYSTEMS a) NEUTRALIZE ACIDS AND BASES b) AN IMPORTANT EXAMPLE OF HOMEOSTASIS c) THEY CAN ACT AS H+ DONORS OR ACCEPTORS d) YOU HAVE BUFFERS IN YOUR BLOOD THAT ALLOW YOU TO MAINTAIN CONSTANT pH e) ANTACIDS ARE EXAMPLES THE CHEMISTRY OF LIFE BIOCHEMISTRY- THERE ARE 4 MAJOR CLASSES OF BIOLOGICALLY ACTIVE MOLECULES a) CARBOHYDRATES (SUGARS OR SACCHARIDES) I. OFTEN POLYMERS OF GLUCOSE II. THE MAJOR AND 1ST SOURCE OF ENERGY IN LIVING THINGS b) LIPIDS (FATS) I. ARE THE MOST DENSE ENERGY STORAGE MOLECULES II. FORM ALL CELL MEMBRANES c) PROTEINS I. POLYMERS OF AMINO ACIDS JOINED BY PEPTIDE BONDS II. ALL ENZYMES ARE PROTEIN d) NUCLEIC ACIDS I. FOUND IN THE NUCLEUS AS DNA OR RNA II. POLYMERS OF NUCLEOTIDES THE CHEMISTRY OF LIFE CARBOHYDRATES I. COMMONLY CALLED SUGARS OR SACCHARIDES (-OSEs) II. CAN BE FOUND AS MONO, DI, OR POLYSACCHARIDES a) MONOSACCHARIDE- GLUCOSE b) DISACCHARIDE- SUCROSE, LACTOSE c) POLYSACCHARIDE- STARCH, CELLULOSE, GLYCOGEN III. THE 1ST SOURCE OF ENERGY IN LIVING THINGS IV. FORM STRUCTURAL ELEMENTS IN ORGANISM LIKE THE CELL WALL a) WOOD IS CELLULOSE b) A BUGS SHELL IS MADE OF CHITIN LIPIDS I. POLYMERS OF FATTY ACIDS II. ARE USED FOR LONG TERM ENERGY STORAGE a) EXAMPLES CHOLESTEROL III. FORM ALL CELL MEMBRANES a) PHOSPHOLIPID b) PREVENTS THE MOVEMENT OF H2O IN OR OUT OF THE CELL IV. ACT AS HORMONES a) TESTOSTERONE & ESTROGEN PROTEINS a) POLYMERS OF AMINO ACIDS JOINED BY PEPTIDE BONDS b) ALL ENZYMES ARE PROTEIN c) PROTEINS HAVE IMPORTANT ROLES AS I. ENZYMES (-ASEs) EXAMPLE AMYLASE, HYDROLASE, ATPase II. HORMONES- like INSULIN, SEROTONIN III. STRUCTUAL ELEMENTS- KERATIN, COLLAGEN NUCLEIC ACIDS I. CARRY HEREDITARY INFORMATION IN THE FORM OF GENES II. FOUND IN THE NUCLEUS a) FORMS AN ALPHA DOUBLE HELIX III. POLYMERS OF NUCLEOTIDES a) CONTAIN A SUGAR –RIBOSE OR DEOXYRIBOSE b) NITROGENOUS BASE c) PHOSPHATE GROUP THE CHEMISTRY OF LIFE MATTER AND ENERGY TRANSFORMATIONS 1. CHEMICAL REACTIONS ARE SYMBOLIZED IN EQUATIONS REACTANTS------PRODUCTS A + B -------------- C + D 1. CHEMICAL REACTIONS STORE AND RELEASE ENERGY a) ENDOTHERMIC REACTIONS–NEED ENERGY TO PROCEED b) EXOTHERMIC REACTIONS – GIVE OFF ENERGY AS THEY PROCEED 2. ACTIVATION ENERGY IS REQUIRED FOR A CHEMICAL REACTION TO PROCEED 3. ENZYMES ARE BIOLOGICAL CATALYSTS THAT LOWER ACTIVATION ENERGY a) THIS SPEED CHEMICAL REACTIONS b) THIS ALLOWS THE CHEMICAL REACTIONS NECESSARY FOR LIFE ENZYMES ARE BIOLOGICAL CATALYSTS ACTIVATION ENERGY IS REQUIRED FOR A CHEMICAL REACTION TO PROCEED ENZYMES ARE BIOLOGICAL CATALYSTS 1. ENZYMES ARE PROTEINS WITH A SPECIFIC 3DIMENSIONAL STRUCTURE 2. ENZYMES LOWER ACTIVATION ENRGY BY BINDING SUBSTRATES AT THEIR ACTIVE SITES 3. ENZYMES CATALYZE REACTIONS WITHOUT BEING CHANGED OR USED UP 4. ENZYME ACTIVITY CAN BE INFLUENCED BY THE ENVRONMENT a) CHANGES IN pH b) CHANGES IN TEMPERATURE c) CHANGES IN ENZYME OR SUBSTRATE CONCENTRATION CHAPTER 7 CELLULAR STRUCTURE THE DISCOVERY OF CELLS 1. (1665) ROBERT HOOK: “CELLS” 2. (1695) ANTON VON LEEUWENHOEK: “ANIMACULES” 3. SCHLIEDEN: PLANTS ARE MADE OF CELLS 4. SCHWANN: ANIMALS ARE MADE OF CELLS 5. VIRCHOW: ALL CELLS COME FROM PREXISTING CELLS CHAPTER 7 CELLULAR STRUCTURE THE CELL THEORY 1. ALL LIVING THINGS ARE MADE OF ONE OR MORE CELLS” 2. CELLS ARE THE BASIC UNIT OF STRUCTURE AND FUNCTION 3. ALL CELLS COME FROM PREXISTING CELLS CELL BIOLOGISTS USE MICROSCOPES 1. THIS IS A COMPOUND LIGHT MICROSCOPE 1. COMPOUND DUE TO MULTIPLE LENSES 2. LIGHT MUST PASS THROUGH THE OBJECT BEING OBSERVED 3. THE EYEPIECE OR OCULAR LENS IS ON TOP 4. THE OBJECTIVE LENSES ARE DOWN NEAR THE OBJECT OTHER TYPES OF MICROSCOPES 1. ELECTRON MICROSCOPE a) GIVES EXTREMELY HIGH MAGNIFICATION AND RESOLUTION 2. SCANNING TUNNELING MICROSCOPE a) GIVES EXTREMELY HIGH MAGNIFICATION AND 3D IMAGES CELL SIZE AND SHAPE 1. A High surface to Volume ratio 2. THE Greater the SURFACE AREA, more stuff gets in and out of cell 3. Greater the VOLUME, less stuff gets in or out; cell starves or is poisoned 4. Cell size varies with function CELL STRUCTURE 3 MAJOR PARTS OF CELL 1. PLASMA MEMBRANE: a) CONTROLS PASSAGE OF MATERIALS IN OR OUT OF CELL 2. NUCLEAR REGION: a) CONTROLS CELLS ACTIVITIES; b) CONTAINS DNA & RNA 3. CYTOPLASM : a) ORGANELLES AND CYTOSOL INTERNAL ORGANIZATION & TYPES OF CELLS 1. EACH CELL CONTAINS “LITTLE ORGANS” CALLED ORGANELLES 2. EACH ORGANELLE PERFORMS SPECIFIC FUNCTIONS FOR THE CELL 3. EUKARYOTIC CELLS: CELLS WITH A NUCLEUS AND OUTER CELL MEMBRANE WHICH ALLOWS MOLECULES TO PASS IN AND OUT 4. PROKARYOTIC CELLS: CELLS WITHOUT A NUCLEUS OR OTHER ORGANELLES ORGANIZATION OF CELLS 1. CELLS ARE ORGANIZED INTO TISSUES a. NERVE TISSUE IS COMPOSED OF ASTROCYTES AND NEURONS b. TISSUES ARE ORGANIZED INTO ORGANS c. THE HEART IS MADE OF MUSCLE AND CONNECTIVE TISSUE d. ORGANS ARE ORGANIZED INTO ORGAN SYSTEMS e. THE RESPIRATORY SYSTEM INCLUDES THE LUNGS, TRACHEA, NASAL PASSAGES, DIAPHRAGM MUSCLE AND BLOOD VESSELS f. ORGANS SYSTEMS ARE ORGANIZED INTO AN ORGANISM g. WE ARE COMPOSED OF OUR BODY SYSTEMS INCLUDING;CARDIOVASCULAR, RPRODUCTIVE, DIGESTIVE, NERVOUS…ETC. PLANT & ANIMAL CELL ORGANELLES 1. CYTOPLASM a) ALL THE AREA BETWEEN THE CELL MEMBRANE AND THE NUCLEUS AND CONTAINS ALL OF THE ORGANELLES WITHIN THE CELL b) RIBOSOMES c) MAKE PROTEINS FROM AA; FOUND ON ER OR IN CYTOSOL d) MADE IN THE NUCLEOLUS OF RRNA e) ROUGH ER f) MAKES PROTEINS AND TRANSPORTS THEM TO OTHER PARTS OF THE CELL VIA VESICLES (LITTLE PACKAGES) 2. RIBOSOMES a) MAKE PROTEINS FROM AMINO ACIDS (AA); b) SYNTHESIZED IN NUCLEOLUS c) FOUND ON ER OR IN CYTOSOL. ROUGH ENDOPLASMIC RETICULUM (ER) – a) MAKES PROTEINS AND TRANSPORTS THEM TO OTHER PARTS OF THE CELLS VIA VESICLES 4. SMOOTH ER a) TRANSPORTS PROTEINS b) SYNTHESIZES LIPIDS 5. GOLGI APPARATUS a) FOUND CLOSE TO ER. b) TAKES PROTEIN FROM ER AND MODIFIES THEM TO MAKE THEM WORK; c) SENDS THEM ON THEIR WAY (MAILROOM OF THE CELL) 6. MITOCHONDRION a) POWERHOUSE OF THE CELL. TAKES IN FOOD; CONVERTS IT TO ATP, WHICH IS BROKEN DOWN FOR ENERGY. b) SOME CELLS HAVE MORE MITOCHONDRIA THAN OTHERS c) CRISTAE: FOLDS IN THE INNER MEMBRANE OF MITOCHONDRIA TO INCREASE SURFACE AREA 7. NUCLEUS a) CONTROLS AND COORDINATES CELL’S ACTIVITIES. CONTAINS CHROMATIN (DNA). DNA CONVERTED TO RNA AND STORED IN NUCLEOLUS. RNA SENT OUT TO CELL AS A MESSENGER b) SURROUNDED BY NUCLEAR ENVELOPE c) NUCLEAR PORES ALLOW RNA TO LEAVE NUCLEUS 8. CYTOSKELETON: SCAFFOLDING THAT GIVES CELL SHAPE a) MICROFILAMENTS: USED FOR MUSCLE CONTRACTION b) MICROTUBULES: THICKER, MOVE ORGANELLES c) CILIA AND FLAGELLA I. HELP SOME CELLS MOVE AROUND II. LINING OF RESPIRATORY TRACT 9. LYSOSOMES: a) CONTAIN DIGESTIVE ENZYMES b) BREAKS DOWN GLUCOSE c) CAN RUPTURE AND KILL CELL 3. CHAPTER 8 CELLULAR TRANSPORT STRUCTURE OF PLASMA MEMBRANE 1 SELECTIVELY PERMEABLE ALLOWS SOME MOLECULES IN; KEEPS OTHERS OUT 2 PHOSPHOLIPID BILAYER POLAR HEADS ON OUTSIDE AND INSIDE OF CELL; NONPOLAR TAILS ON INSIDE OF MEMBRANE 3 POLAR = HYDROPHILIC = WATER LOVING 4 NONPOLAR = HYDROPHOBIC = WATER HATING MOLECULES ON THE PLASMA MEMBRANE CARBOHYDRATES ACT AS RECEPTORS; MOLECULES ATTACH TO CELL; TELL IT WHAT TO DO CHOLESTEROL GIVES MEMBRANE SHAPE; RIGIDITY RECEPTOR PROTEINS BIND HORMONES & OTHER SUBSTANCES COMING IN FROM OUTSIDE THE CELL RECOGNITION PROTEINS PROTEINS ON MEMBRANE THAT ARE USED FOR RECOGNITION BY EXTRA-CELLULAR SUBSTANCES ADHESION PROTEINS HELP CELLS OF A CERTAIN TYPE STICK TOGETHER TO FORM TISSUES WHAT GETS IN AND OUT OF THE CELL MEMBRANE? HOW DO SOME SUBSTANCES PASS THROUGH THE MEMBRANE WHILE OTHERS STAY OUT? MATERIALS MOVES ACROSS THE MEMBRANE IN TWO WAYS 1. PASSIVE TRANSPORT 2. ACTIVE TRANSPORT PASSIVE TRANSPORT DIFFUSION MOVEMENT OF MOLECULES FROM HIGH TO LOW CONCENTRATION OXYGEN, CO2, FAT SOLUBLE MOLECULES, AND WATER PASS THROUGH THE MEMBRANE BY DIFFUSION ACTIVE TRANSPORT ACTIVE TRANSPORT USING ENERGY TO TRANSPORT LARGE MOLECULES INTO THE CELL AGAINST THEIR CONCENTRATION GRADIENTS 1. CELL MEMBRANE PUMPS CARRIER PROTEINS PUMP IONS AGAINST THEIR CONCENTRATION GRADIENT 2. ENDOCYTOSIS ENGULFING OF LARGE PARTICLES OR LIQUIDS BY PLASMA MEMBRANE CELLS TAKE IN CHOLESTEROL BY ENDOCYTOSIS FROM BLOODSTREAM PINOCYTOSIS ENDOCYTOSIS OF LIQUID 3. EXOCYTOSIS VESICLE CONTENTS EXPELLED BY CELL PROTEINS ARE TRANSPORTED BY EXOCYTOSIS EXOCYTOSIS ANIMATION ENDOCYTOSIS ANIMATION OSMOSIS OSMOSIS DIFFUSION OF WATER ISOTONIC SOLUTION CELLS ARE AT EQUILIBRIUM; NO NET MOVEMENT OF WATER HYPERTONIC SOLUTION CELL IS IN A SOLUTION THAT HAS LOTS OF SALTS OR OTHER IONS IN IT; WATER RUSHES OUT OF THE CELL AND THE CELL SHRINKS HYPOTONIC SOLUTION CELL IS IN A SOLUTION THAT HAS LITTLE OR NO SALTS OR OTHER IONS IN IT; WATER RUSHES INTO THE CELL AND THE CELL SWELLS BIOLOGY CH9 CELLULAR RESPIRATION & PHOTOSYNTHESIS PHOTOSYNTHESIS & CELLULAR RESPIRATION PHOTOSYNTHESIS + SUNLIGHT CO2 + H20 C6H12O6 + O2 THE BASIC EQUATIONS CELLULAR RESPIRATION C6H12O6 + O2 CO2 + H20 + ATP OCCURS IN THE CHLOROPLAST OF AUTOTROPHS OCCURS IN THE MITOCHONDRIA OF ALL CELLS* PHOTOSYNTHESIS + SUNLIGHT CO2 + H20 C6H12O6 + O2 OCCURS IN THE CHLOROPLAST OF AUTOTROPHS HAS BOTH LIGHT AND DARK REACTIONS The LIGHT Reactions + SUNLIGHT CO2 + H20 C6H12O6 + O2 OCCURS IN THE THYLAKOIDS OF CHLOROPLAST LIGHT REACTIONS- Use light to split H20, make O2, and a Hydrogen Ion gradient which makes ATP and NADPH Also known as the LIGHT DEPENDENT REACTIONS The DARK Reactions + ATP + NADPH CO2 + H20 C6H12O6 + O2 OCCURS IN THE STROMA OF CHLOROPLAST The Dark Reactions/Calvin Cycle: Use CO2 , (+ ATP + NADPH) to make C6H12O6 !!! Also known as the LIGHT INDEPENDENT REACTIONS AND OR CALVIN CYCLE!! CELLULAR RESPIRATION OVERVIEW ALL CELLS CELLULAR RESPIRATION Has a few Reactions Glycolysis The Krebs/TCA/Citric Acid Cycle The Electron Transport Chain C6H12O6 + O2 CELLULAR RESPIRATION: Glycolysis GLYCOLYSIS C6H12O6 + O2 2ATP + H2O + 2Pyruvate OCCURS IN THE CYTOPLASM!!! NOT MITOCHONDRIA CO2 + H20 + ATP OCCURS IN THE MITOCHONDRIA OF OR Fermentation (if there’s no O2 available) And 2 Major Pathways Aerobic Reactions – use O2 Anaerobic Reactions – Doesn't use O2 ANAEROBIC REACTION -Doesn’t Use O2 In Short: Step 1 Glycolysis (glucose-lysis) C6H12O62Pyruvate + H2O +2ATP CELLULAR RESPIRATION: THE KREBS CYCLE(*aka. TCA/Citric Acid Cycle) 2 Pyruvate + O2 2ATP + CO2 +2NADH + 2FADH2 AEROBIC REACTION –USES O2 OCCURS IN THE MATRIX of MITOCHONDRIA In Short: Step 2 The Krebs/TCA Cycle 2 Pyruvate CO2 + 2ATP + 2NADH + 2FADH2 CELLULAR RESPIRATION: The ELECTRON TRANSPORT CHAIN The Electron Transport Chain 2NADH+ 2FADH2 H20 + 32-34 ATP OCCURS ON THE CRISTAE OF THE MITOCHONDRIA Uses a Hydrogen Ion gradient to make ATP! In Short: Step 3 The Electron Transport Chain 2NADH + 2FADH2 H20 + 32-34ATP CELLULAR RESPIRATION: if there’s no O2 Organisms use Glycolysis then Fermentation!! ANAEROBIC RESPIRATION- AKA- FERMENTATION 2 TYPES In Animals In Short: Under Anaerobic Conditons 2 Pyruvate + 2ATP + Lactic Acid Step 1: Glycolysis In Yeast Step 2: Fermentation 2 Pyruvate + 2ATP + Ethanol CHAPTER 10-11 CELL DIVISION WHY DO CELLS DIVIDE? 1. THEY HAVE GROWN TOO LARGE SO…. THE SURFACE AREA/VOLUME RATIO IS TOO SMALL 2. TO ALLOW AN ORGANISM TO GROW AND INCREASE ITS SIZE 3. CELLS HAVE DIED AND NEED TO BE REPLACED DNA IN THE CELL INSIDE THE NUCLEUS DNA CONDENSES TO FORM CHROMOSOMES CHROMOSOMES ARE MADE FROM PROTEINS CALLED HISTONES AND DNA TOGETHER KNOWN AS CHROMATIN CHROMATIN ALLOWS DNA TO COIL INTO NUCLEOSOMES EACH CHROMOSOME CONSISTS OF IDENTICAL HALVES CALLED SISTER CHROMATIDS SISTER CHROMATIDS ARE JOINED TOGETHER AT THE CENTROMERE NUMBER & TYPES OF CHROMOSOMES SEX CHROMOSOMES DETERMINE THE GENDER OF AN ORGANISM XX = FEMALE XY = MALE DIPLOID CELLS: CELLS WITH 2 COPIES OF EACH CHROMOSOME THE NORMAL BODY CELLS ARE CALLED SOMATIC CELLS, AND THEY ARE ALL DIPLOID DIPLOID & HAPLOID CELLS CELL DIVISION IN PROKARYOTES PROKARYOTIC ORGANISMS: AUNICELLULAR BACTERIA WITH NO NUCLEUS OR MEMBRANE BOUND ORGANELLES THEIR DNA IS FOUND AS ONE CIRCULAR CHROMOSOME THEIR CELLS REPRODUCE THROUGH BINARY FISSION DNA IS REPLICATED, CELL DOUBLES IN SIZE AND SPLITS STAGES OF MITOSIS PROPHASE CHROMATIN CONDENSES & NUCLEUS DISAPPEARS SPINDLE FIBERS (MICROTUBULES) FORM & MOVE CHROMOSOMES CENTROSOMES BEGIN TO MIGRATE METAPHASE SPINDLES ALIGN CHROMOSOMES IN THE MIDDLE OF THE CELL AKA METAPHASE PLATE ANAPHASE CENTROMERES ARE PULLED APART BY SPINDLE FIBERS AND SISTER CHROMATIDS SPLIT CHROMATIDS MOVE TOWARD OPPOSITE POLES TELOPHASE CHROMOSOMES ARRIVE AT OPPOSITE ENDS OF CELL NUCLEUS REAPPEARS SPINDLE DISAPPEARS CYTOKINESIS CELL SPLITS TO FORM 2 NEW DAUGHTER CELLS STAGES OF MITOSIS CYTOKINESIS - PLANT VS. ANIMAL CELLS IN ANIMAL CELLS: A CLEAVAGE FURROW PINCHES ONE CELL INTO 2 CELLS MEIOSIS GETTING FROM DIPLOID TO HAPLOID CELLS: MEIOSIS WHERE DOES IT OCCUR IN HUMANS? MALES: TESTES FEMALES: OVARIES MEIOSIS OVERVIEW MEIOSIS I MEIOSIS II 1 DIPLOID * CELL SPLITS INTO 2 HAPLOID CELLS 2HAPLOID DAUGHTER CELLS UNDERGO MITOSIS FFORMS 4 HAPLOID SEX CELLS SSAME AS MITOSIS MEIOSIS I PROPHASE I HOMOLOGOUS CHROMOSOMES PAIR TOGETHER A PROCESS CALLED SYNAPSIS EACH HOMOLOGOUS PAIR OF CHROMOSOMES IS CALLED A TETRAD PORTIONS OF CHROMATIDS BREAK OFF AND ATTACH TO ADJACENT HOMOLOGOUS CHROMATIDS THIS PROCESS IS KNOWN AS CROSSING OVER CROSSING OVER CREATES NEW GENE COMBINATIONS – THE CHROMOSOMES OF YOUR SEX CELLS ARE COMBINATIONS OF BOTH YOUR MOM AND DADS!!! METAPHASE I HOMOLOGOUS CHROMOSOMES LINE UP IN THE MIDDLE OF THE CELL ANAPHASE I HOMOLOGOUS CHROMOSOMES MOVE TO OPPOSITE POLES OF THE CELL RANDOM SEPARATION OF HOMOLOGOUS CHROMOSOMES IS CALLED INDEPENDENT ASSORTMENT TELOPHASE I CHROMOSOMES REACH OPPOSITE POLES OF THE CELL CYTOKINESIS BEGINS MEIOSIS I MEIOSIS II 2 HAPLOID CELLS GO THROUGH THE PROCESS OF MITOSIS & CELL DIVISION THE RESULT OF MEIOSIS II IS 4 HAPLOID CELLS GAMETE FORMATION IN MALES MEIOSIS CREATES 4 SPERM CELLS (CALLED SPERMATIDS) IN FEMALES THE CYTOPLASM IS UNEVENLY DIVIDED SO THAT ONLY 1 BIG CELL THE OVUM IS FORMED, ALONG WITH 3 OTHER CELLS KNOWN AS POLAR BODIES MEIOSIS II MEIOSIS VOCABULARY • DIPLOID CELLS CELLS WITH 2 OF EACH TYPE OF CHROMOSOME (1 FROM MOM AND 1 FROM DAD) • N= NUMBER OF PAIRS OF CHROMOSOMES • DIPLOID CELLS = 2N • ZYGOTE = FERTILIZED EGG • GAMETES= HAPLOID SEX CELLS • FERTILIZATION= UNION OF EGG AND SPERM • SEXUAL REPRODUCTION PARENTS GENERATE SPECIALIZED SEX CELLS