HL3340 Library Depost patient numbers
advertisement

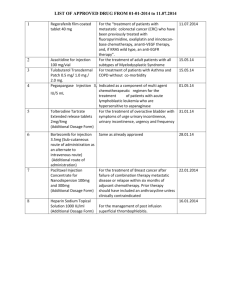
Lord Avebury MP (NONE) To ask Her Majesty’s Government which drugs have been delisted from the Cancer Drugs Fund; how many patients are currently being treated with each drug; and what steps they are taking to develop a new system for prescribing each drug. HL3340 NHS England publishes information on the number of patient applications for particular drugs/indications contained on the national CDF list on a quarterly basis. This information also includes the number of applications approved through the individual CDF request process. The latest information is set out in Tables 1-3. It is also available at: www.england.nhs.uk/ourwork/pe/cdf/. Table 1: Notifications - patient numbers and invoiced spend Drug Abiraterone Albumin bound paclitaxel Axitinib Bendamustine CDF indication 1st line treatment of metastatic castrate resistant prostate cancer (Mcrpc) in adult men who are symptomatic, or mildly symptomatic, after failure of androgen deprivation therapy (ADT) in whom chemotherapy is not yet clinically indicated The first line treatment of advanced adenocarcinoma of the pancreas in combination with gemcitabine Option for 2nd line advanced renal cell carcinoma with progression after TKI or a cytokine (until 27th May 2015) 2nd or subsequent line treatment of CLL for patients whom fludarabine combination therapy is not a therapeutic option Treatment of relapsed low grade NHL in patients unable to receive standard chemotherapy 1st line treatment of low grade non-hodgkins lymphoma in combination with rituximab 2nd and subsequent line treatment of mantle cell lymphoma in patients who have not received previous bendamustine 1st line treatment of mantle cell lymphoma in combination with rituximab in patients unsuitable for standard first line treatment Treatment of relapsed multiple myeloma where other treatments are not appropriate Total number of application received for each indication Apr-15 Total number of application received for each indication May-15 Total number of application received for each indication Jun-15 Total number of application received for each drug 80 70 86 236 41 40 46 127 34 36 34 104 9 20 20 25 47 33 57 46 83 438 4 3 3 10 5 11 17 27 18 Bevacizumab Treatment of patients with triple negative metastatic breast cancer and/or prior taxane therapy 11 16 18 1st line treatment recurrent or metastatic cervical cancer 16 19 25 36 33 48 2nd line treatment of metastatic colorectal cancer in combination with standard chemotherapy in patients who have not previously received bevacizumab. Only to be administered concurrently with chemotherapy, not as single agent maintenance therapy. 3rd line treatment of metastatic colorectal cancer in combination with standard chemotherapy in patients who have not previously received bevacizumab. Only to be administered concurrently with chemotherapy, not as single agent maintenance therapy The third line treatment of low grade gliomas of childhood Bortezomib 1st line treatment of advanced (stage IIIc/IV) ovarian cancer, suboptimally debulked either at primary or delayed primary (interval) surgery (including peritoneal and fallopian tube cancer) OR unsuitable for debulking surgery Treatment of relapsed or refractory multiple myeloma at second and subsequent relapse in patients who are bortezomib naïve and where the patient unable to access bortezomib at first relapse Treatment of chronic phase CML refractory to nilotinib or dasatinib Bosutinib Brentuximab Treatment of accelerated phase CML refractory to nilotinib or dasatinib Treatment of chronic phase CML where there is significant intolerance to dasatinib and nilotinib Treatment of accelerated phase CML where there is significant intolerance to dasatinib and nilotinib Treatment of relapsed or refractory Hodgkins lymphoma in patients who have failed at least two prior multi-agent chemotherapy regimens and are not ASCT candidates Treatment of relapsed/ refractory anaplastic large cell lymphoma where no other salvage treatment is available (from 12/01/2015) 432 9 4 11 1 0 2 56 64 63 11 8 10 6 3 6 0 0 0 3 5 1 0 0 0 14 25 13 29 24 62 5 1 4 Cabazitaxel Cabozantinib Cetuximab Clofarabine Crizotinib Dasatinib Treatment of castrate-resistant metastatic prostate cancer in patients who have had previous treatment with docetaxel based regimens (from 22/05/2015) Treatment of progressive, unresectable locally advanced or metastatic medullary thyroid carcinoma 1st line treatment of metastatic and/or recurrent squamous cell carcinoma of the head and neck 1st Line treatment of metastatic coloreactal cancer in combination with the following regimens: FOLFOX4 or FOLFOX6 or OxMdG Chemotherapy (From 13/02/2014) 1st line treatment of metastatic colorectal cancer in combination with Irinotecan based chemotherapy (From 13/02/2014) 3rd and subsequent line treatment of metastatic colorecal cancer as a single agent (From 13/02/2014) 3rd and subsequent line treatment of metastatic colorecal cancer as a single agent in patients not treated to progression under NICE TA176 (From 13/02/2014) Acute myeloblastic leukaemia in patients with relapsed/refractory disease in whom the intent is to use treatment as a bridge to bone marrow transplantation Acute lymphoblastic leukaemia in patients with relapsed/refractory disease in whom the intent is to use treatment as a bridge to bone marrow transplantation In second and subsequent line anaplastic lymphoma kinase (ALK)-positive advanced nonsmall lung cancer Treatment of adults with Philadelphia chromosome positive (Ph+) acute lymphoblastic leukaemia (ALL) with resistance or intolerance to prior therapy including Imatinib (from 12/01/2015). Treatment of chronic phase CML which is refractory or significant intolerance to imatinib and intolerance to nilotinib Treatment of accelerated phase CML which is refractory or significant intolerance to imatinib and intolerance to nilotinib 0 4 41 45 1 0 0 1 23 18 20 4 6 10 69 79 104 29 34 37 9 3 5 3 4 5 450 16 2 0 2 8 14 12 2 1 5 34 29 9 5 7 0 0 0 Enzalutamide Eribulin Everolimus Ibrutinib Idelalisib Lenalidomide Nelarabine The treatment of chemotherapy naïve castrate-resistant Metastatic Prostate Cancer Treatment of patients with locally advanced or metastatic breast cancer who have been previously treated with (or unsuitable for) an anthracycline, a taxane and capecitabine 2nd line treatment of metastatic renal cell carcinoma where disease has progressed on or after treatment with VEGFtargeted therapy or where patient has a contra-indication to or is intolerant of VEGF targeted therapy 1st or 2nd line treatment of unresectable or metastatic, moderately differentiated neuroendocrine tumours of pancreatic origin in adults with progressive disease (from 12/01/2105) 2nd line treatment of hormone receptor +ve, HER2 negative advanced breast cancer, in combination with exemestane, in post menopausal women without symptomatic visceral disease after recurrence or progression following a non steroidal aromatase inhibitor who have not received previous exemestane 234 204 223 661 55 68 112 235 14 11 15 4 3 2 175 35 39 52 Treatment of relapsed/ refactory chronic lymphocytic leukaemia 56 55 65 Treatment of relapsed/ refractory mantle cell lymphoma 26 18 23 10 14 19 23 19 20 In combination with rituximab for the treatment of adult patients with relapsed chronic lymphocytic leukaemia (CLL) not eligible for cytotoxic therapies 2nd line treatment of multiple myeloma in patients who have contraindications to the use of bortezomib Transfusion-dependent anaemia due to low- or intermediate-1-risk myelodysplastic syndromes associated with an isolated deletion 5q plus one other cytogenetic abnormality when other therapeutic options are insufficient or inadequate (from 19/01/2015) Treatment of refractory T-cell lymphoblastic non-Hodgkin’s lymphoma as a bridge to bone marrow transplantation 243 43 65 1 0 2 0 2 0 6 Ofatumumab Panitumumab Pegylated Liposomal Doxorubicin Treatment of refractory T cell acute lymphoblastic leukaemia as a bridge to bone marrow transplantation In combination with chlorambucil for the treatment of patients with CLL who have not received prior therapy and who are not eligible for fludarabine-based therapy (removed 08/09/2015) 1st Line treatment of metastatic coloreactal cancer in combination with the following regimens: FOLFOX4 or FOLFOX6 or OxMdG Chemotherapy 1st Line treatment of metastatic coloreactal cancer in combination with irinotecan based chemotherapy (from 04/09/2015) 3rd and subsequent line treatment of metastatic colorecal cancer as a single agent 3rd and subsequent line treatment of metastatic colorecal cancer as a single agent in patients not treated to progression under NICE TA176 1st line treatment of sarcoma in patients with cardiac impairment who need an anthracycline 2nd line treatment of sarcoma in patients with cardiac impairment who need an anthracycline 2nd line treatment of fibromatosis Pemetrexed 2nd line treatment of patients with locally advanced or metastatic nonsmall cell lung cancer other than predominantly squamous cell histology in patients who did not receive 1st line pemetrexed e.g. 1st line clinical trial (removed 21/07/2015) Maintenance treatment of stage IIIB/IV non-squamous non-small cell lung cancer after response to pemetrexed-containing first line therapy 0 1 3 7 2 3 6 6 6 0 0 0 12 51 6 5 12 2 5 3 1 0 0 2 2 1 0 0 0 10 3 13 6 144 26 33 59 Peptide receptor Radionuclide Therapy to include Lutetium177 Octreotate or Yttrium90 octreotide/ octreotate Treatment of advanced neuroendocrine tumours, i.e. for pNETS after sunitinib/Chemotherapy, For midgut Carcinoid, after octreotide/somatostatin therapies 20 11 11 42 Pertuzumab In combination with docetaxel and trastuzumab for the first line treatment of locally advanced or metastatic HER2+ breast cancer 78 69 68 215 Pomalidomide Ponatinib Radium-223 Dichloride Regorafenib Ruxolitinib Sorafenib Sunitinib Treatment of relapsed and refractory multiple myeloma who have received at least two prior treatment regimens, including both lenalidomide and bortezomib, and have demonstrated disease progression on the last therapy Philadelphia-chromosome-positive acute lymphoblastic leukaemia (Ph+ ALL) with the T315I mutation Chronic Myeloid Leukaemia with T315I Mutation Treatment of castration-resistant prostate cancer patients with bone metastases Treatment of adult patients with advanced (metastatic or unresectable) gastro-intestinal stromal tumours (GIST) after failure of at least previous imatinib and sunitinib 1st line treatment of symptomatic splenomegaly in adult patients with primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. For new patients only. 2nd line treatment of symptomatic splenomegaly in adult patients with primary myelofibrosis, post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. For new patients only. 1st line treatment of advanced hepatocellular carcinoma in patients with Childs A disease 1st line treatment of advanced hepatocellular carcinoma in patients with low disease burden Childs B disease Treatment of papillary or follicular thyroid cancer that is inoperable or metastatic disease that’s is refractory to radioiodine 1st line treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumour with disease progression 2nd line treatment of unresectable or metastatic, welldifferentiated pancreatic neuroendocrine tumour with disease progression 3rd line treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumour with disease progression 63 57 72 0 1 0 192 9 0 3 5 64 61 84 209 2 4 6 12 18 22 22 99 18 11 8 35 31 45 10 2 8 1 2 6 2 1 0 4 0 1 2 1 0 140 11 1st line treatment of patients with advanced renal cell carcinoma (RCC) who have at least three of six prognostic risk factors Treatment of HER2-positive locally advanced/unresectable or metastatic (Stage IV) breast cancer who previously received trastuzumab and a taxane, separately or in combination Treatment of symptomatic, locally advanced (unresectable) or metastatic medullary thyroid cancer. Symptomatic metastatic basal cell carcinoma (BCC), or locally advanced BCC inappropriate for surgery or radiotherapy Total Temsirolimus Trastuzumab Emtansine Vandetinib Vismodegib 3 2 2 7 46 46 52 144 6 2 4 12 9 3 9 21 1503 1459 1819 4781 Source: NHS England: Quarterly figures – including notifications and Individual Cancer Drug Fund Requests (ICDFRs) Table 2: Molecular diagnostic tests - patient numbers and invoiced spend To define eligibility for treatment with Cetuximab or Panitumumab in the treatment of colorectal cancer Total RAS Testing 388 251 422 1061 388 251 422 1061 Source: NHS England: Quarterly figures – including notifications and Individual Cancer Drug Fund Requests (ICDFRs) Table 3: Individual Cancer Drug Fund Requests (ICDFRs) - Region and NHS England by month Region London Midlands North South Month Number of applications received for consideration by screening panel Number of applications put forward for consideration by CDF panel Number of applications approved by CDF panel Number of CDF panel decisions communicated back to requester within 10 working days Number of applications refused by CDF panel Apr-15 4 1 0 1 1 May-15 12 5 3 2 5 Jun-15 6 1 0 1 1 TOTAL 22 7 3 4 7 Apr-15 7 2 0 2 2 May-15 14 4 0 4 4 Jun-15 16 5 3 2 5 TOTAL 37 11 3 8 11 Apr-15 6 5 2 3 5 May-15 5 3 2 1 3 Jun-15 8 4 2 2 4 TOTAL 19 12 6 6 12 Apr-15 7 2 2 0 2 May-15 9 1 1 0 1 Jun-15 10 1 1 0 1 TOTAL 26 4 4 0 4 NHSE TOTAL 104 34 16 18 34 Source: NHS England: Quarterly figures – including notifications and Individual Cancer Drug Fund Requests (ICDFRs)