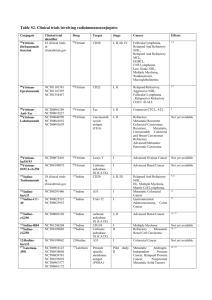
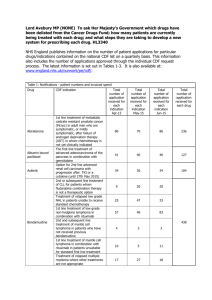
Table S1. Clinical trials involving chemical immunoconjugates
advertisement

Table S1. Clinical trials involving chemical immunoconjugates Conjugate Clinical trial identifier NCT01100502 NCT00848926 NCT01352520 NCT00866047 NCT00430846 NCT00947856 NCT01026415 NCT01060904 NCT01309789 NCT01026233 NCT01100502 NCT00704158 NCT00412828 NCT01156753 Drug Target Stage Cancer Effects monometil auristatin E (MMAE) CD30 I, II, III Relapsed or Refractory HL, Relapsed or Refractory ALCL, Relapsed or Refractory NHL, Mycosis Fungoides, Extensive Lymphomatoid Papulosis (LyP) 1-4 monomethyl auristatin E (MMAE) GPNMB I, II 4, 5 ASG-5ME NCT01228760 NCT01166490 SLC44A4 (AGS-5) I BAY79-4620 NCT01028755 NCT01065623 Advanced Solid Tumors Not yet available NCT01015911 Carbonic anhydrase IX (CAIX) CD70 I SGN-75 I NCT01114230 AGS-16 I Relapsed or Refractory NHL, Metastatic Renal Cell Carcinoma Advanced Renal Cell Carcinoma Not yet available AGS-16M8F Trastuzumab emtansine NCT00932373 NCT00679341 NCT00875979 NCT00928330 NCT00951665 NCT00829166 NCT01120561 NCT00943670 NCT00679211 NCT00509769 NCT00781612 NCT01120184 NCT00721669 monomethyl auristatin E (MMAE) monomethyl auristatin E (MMAE) monomethyl auristatin F (MMAF) monomethyl auristatin F (MMAF) maytansinoid DM1 Locally Advanced or Metastatic Breast Cancer, Unresectable Stage III or Stage IV Melanoma Advanced Prostate Cancer, Pancreatic Adenocarcinoma HER2 I, II, III Metastatic Breast Cancer 4, 6, 7 maytansinoid DM4 maytansinoid DM4 maytansinoid DM4 Integrin I Solid Tumors 8 CD138 I, II 4 CD33 I maytansinoid DM4 CD19 I SAR566658 NCT00539682 NCT00796731 NCT00549185 NCT01156870 Relapsed/Refractory Multiple Myeloma Recurrent Ovarian, Prostate, Colorectal or Non-Small Cell Lung Cancers Refractory Epithelial Cancer Relapsed/Refractory NHL maytansinoid DM4 CA6 I Not yet available BIIB015 NCT00674947 Cripto I AMG386 NCT00770536 NCT00861419 NCT00807859 NCT01204749 NCT00511459 NCT01253681 NCT01331941 NCT00752570 NCT01281254 maytansinoid DM4 peptide-Fc fusion protein (peptibody) Angiopoietin1 and Angiopoietin2 I, II, III CA6-expressing Cancers (many breast, ovarian, cervical, lung and pancreatic tumors) Relapsed or Refractory Solid Tumors Advanced Recurrent Epithelial Ovarian Cancer, Advanced Solid Tumors, HER2-positive Locally Recurrent or Metastatic Breast Cancer, Recurrent Primary Peritoneal or Fallopian Tube Cancers, Her2-Negative, Metastatic or Brentuximab vedotin (SGN35) CDX-011 (CR011vcMMAE) IMGN388 BT-062 EMD 273066 SAR3419 NCT01001442 NCT00723359 NCT00132522 NCT00016237 Not yet available Not yet available 9 10 Not yet available 11-13 NCT00853372 NCT00583674 NCT00872014 NCT00102830 NCT00479817 NCT00467025 NCT01210222 NCT01249521 NCT01290263 NCT01042379 Locally Recurrent Breast Cancer, Metastatic Colorectal Carcinoma, Metastatic Clear Cell Carcinoma of the Kidney, Metastatic Gastric, Gastroesophageal Junction, or Distal Esophageal Adenocarcinoma, Advanced or Inoperable Hepatocellular Carcinoma, Persistent/Recurrent Carcinoma of the Endometrium, Recurrent Glioblastoma Small Cell Lung Cancer, Relapsed and Relapsed Refractory CD56-Positive Multiple Myeloma, 14, 15 NCT00065429 NCT00346255 NCT00346385 NCT01237678 NCT00991562 NCT00868608 NCT01055496 NCT00867087 NCT00724971 NCT01363297 NCT01232556 NCT00073749 NCT00717925 NCT00299494 NCT01134575 NCT01371630 mertasine CD56 I, II calicheamicin CD22 I, II, III Milatuzumabdoxorubicin (hLL1-DOX ) NCT01101594 Doxorubicin CD74 I, II Multiple Myeloma Not yet available MDX-1203 NCT00944905 CD70 I 47 clinical trials in clinicaltrials.go v VEGF I, II, III Advanced/Recurrent Clear Cell Renal Cell Carcinoma, Relapsed/Refractory B-Cell NHL Relapsed or Refractory Advanced Solid Tumors, Relapsed or Refractory Advanced NHL, Malignant Glioma, Gliosarcoma, Glioblastoma multiforme, Advanced Refractory, Relapsed, or Untreated AML Not yet available Aflibercept DNA alkylating cytotoxic Drug A VEGFR-1 and VEGFR-2 Lorvotuzumab mertansine (BB10901/IMG N901) Inotuzumab ozogamicin (CMC-544) NHL, Relapsed/Refractory Positive DLBCL 4, 16, 17 CD22- 18 AML = Acute Myeloid Leukaemia; ALCL = Anaplastic Large-Cell Lymphoma; DLBCL = Diffuse Large B-Cell Lymphoma; HL = Hodgkin’s Lymphoma; NHL = Non-Hodgkin’s Lymphoma References 1. Deutsch YE, Tadmor T, Podack ER, et al. CD30: an important new target in hematologic malignancies. Leuk Lymphoma 2011. 2. Younes A, Bartlett NL, Leonard JP, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med 2010;363:1812-21. 3. Brentuximab vedotin. Drugs R D 2011;11:85-95. 4. Beck A, Senter PD, Chari RJ. World Antibody Drug Conjugate Summit Europe: February 21-23, 2011; Frankfurt, Germany. MAbs 2011;3. 5. Naumovski L, Junutula JR. Glembatumumab vedotin, a conjugate of an anti-glycoprotein non-metastatic melanoma protein B mAb and monomethyl auristatin E for the treatment of melanoma and breast cancer. Curr Opin Mol Ther 2010;12:248-57. 6. Burris HA. Trastuzumab emtansine: a novel antibody-drug conjugate for HER2-positive breast cancer. Expert Opin Biol Ther 2011;11:807-19. 7. Niculescu-Duvaz I. Trastuzumab emtansine, an antibody-drug conjugate for the treatment of HER2+ metastatic breast cancer. Curr Opin Mol Ther 2010;12:350-60. 8. ImmunoGen. ImmunoGen, Inc. Announces Encouraging IMGN388 Clinical Data Reported at ASCO. 2010. Available at: http://phx.corporate-ir.net/phoenix.zhtml?c=97573&p=irolnewsArticle&ID=1435268&highlight=. 9. Ko YJ, Bubley GJ, Weber R, et al. Safety, pharmacokinetics, and biological pharmacodynamics of the immunocytokine EMD 273066 (huKS-IL2): results of a phase I trial in patients with prostate cancer. J Immunother 2004;27:232-9. 10. Coiffier B, Ribrag V, Dupuis J, et al. Phase I/II study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered weekly to patients with relapsed/refractory B-cell NonHodgkin's Lymphoma (NHL). Asco Annual Meeting. June 2011. abstract 8017. 11. Mita AC, Takimoto CH, Mita M, et al. Phase 1 study of AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clin Cancer Res 2010;16:3044-56. 12. Herbst RS, Hong D, Chap L, et al. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. J Clin Oncol 2009;27:3557-65. 13. Amgen. Updated AMG 386 Data Demonstrate Promising Antitumor Activity in Patients With Recurrent Ovarian Cancer. 2010. Available at: http://wwwext.amgen.com/media/media_pr_detail.jsp?year=2010&releaseID=1480835. 14. ImmunoGen. ImmunoGen, Inc. Announces Presentation of Encouraging Clinical Data for Lorvotuzumab Mertansine at ESMO Annual Meeting. 2010. 15. ImmunoGen. Lorvotuzumab mertansine. 2011. Available at: http://www.immunogen.com/wt/page/IMGN901b. 16. Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: results of a phase I study. J Clin Oncol 2010;28:2085-93. 17. Ogura M, Tobinai K, Hatake K, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci 2010;101:1840-5. 18. Teng LS, Jin KT, He KF, et al. Clinical applications of VEGF-trap (aflibercept) in cancer treatment. J Chin Med Assoc 2010;73:449-56. Table S1: Details of the clinical trials involving chemical immunoconjugates (data gathered from US clinicaltrials.gov, Clinical Trials register EU and available bibliography in sponsor an PubMed websites).