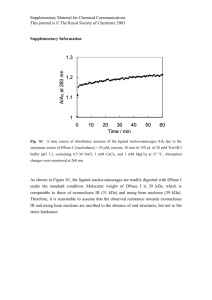
DNase treatment of RNA prep
advertisement

DNase treatment of RNA prep: Materials Needed: 10ul DNaseI and 10ul DNase 10X buffer per sample Phenol/Chloroform (5:1) (Ambion) 100% EtOH. 3M NaOAc (sodium acetate) pH 5.7 (or 5.2) Microcentrifuge (4oC) P-1000, P-200, P-20 pipettors. Procedure: 1. Make up master mix of DNase I and 10X buffer for all samples to be treated. For example, if there are 10 samples (80ul volume), then make a master mix contaning 10ul of both DNase I and 10X buffer for each sample. Make slightly more than is needed to correct for pipetting error. In the sample above, make up a mix for 10.5 reactions = 105 ul 10X Buffer + 105ul DNase I. 2. Pipette appropriate volume master mix into each sample. In the example above, add 20ul master mix to each 80ul sample (1X final concentration). 3. Place tubes @37oC (heat block or water bath) for 30’. 4. Add equal volume of phenol/chloroform (5:1) to each sample (100ul for example above). 5. Shake vigorously or vortex for 15 seconds, then incubate 1’ @RT. 6. Centrifuge @13K rpm, 10’, RT. Label fresh set of tubes. 7. Carefully remove top layer (aqueous phase) and pipet into fresh tubes. 8. Add 1/10 volume NaOAc and 3 volumes EtOH to each sample (a master mix can be prepared ahead of time if desired). 9. Place samples on ice (ice/H2O slurry) for 1-2hrs. Alternatively, the samples can precipitate @ -20oC overnight (this gives much bigger pellets.) 10. Centrifuge @13K rpm, 15’, 4oC. 11. Carefully remove supernatant. 12. Wash pellet with appropriate volume 75% EtOH 13. Centrifuge @ 10k rpm, 5’, 4oC. Carefully remove all traces of super natant wash being careful not to disturb pellet. 14. Let pellet air dry for ~5’. 15. Resuspend pellet in appropriate volume sterile H2O. RNA can be stored at -20oC (short term) or -80oC (long term).