Magnesium and HCl Lab
advertisement

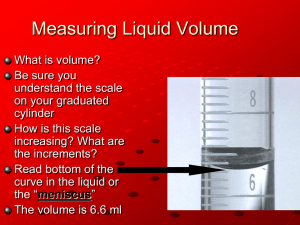
Evidence of Chemical Changes Hydrochloric Acid and Magnesium Safety concern: It is absolutely essential during this lab that you wear safety glasses. You will be working with acid and fire, so there is absolutely no room for any playing around! Work slowly and methodically. Question: Research a little bit about hydrochloric acid and answer all questions in blue: 1. Is hydrochloric acid an ELEMENT or a Chemical COMPOUND? 2. What is its chemical formula? 3. In your own words, what does this chemical formula mean? 4. Is there any place we find hydrochloric acid in the human body? Explain. 5. Why do we need to be careful when using hydrochloric acid in the laboratory? 6. A ‘chemical property’ of a substance is how it reacts with other substances. What could you say is a chemical property of most acids? (in other words, what type of substances do most acids react with) Question: Research a little about Magnesium and answer the questions in blue: 1. Is Magnesium an element or a compound? 2. What is its chemical symbol? 3. Explain, in your own words, what is an element? 4. Explain, in your own words, what is a compound? Lab Procedures: In this lab, you will be adding Magnesium to a small quantity of Hydrochloric Acid, observing and looking for the evidence of a chemical change. Then you will be conducting a second reaction. READ THE ENTIRE LAB FIRST BEFORE DOING ANYTHING!!! Collect the following materials after you put on safety glasses and lab coats: 25 mL graduated cylinder with a clay stopper Strip of Magnesium metal (approximately 1-2 cm in length) Lighters – Share these with other groups. DO NOT light these until necessary!!! If you do, you will be asked to sit down and not participate further in the lab. REACTION #1 A. Carefully measure out approximately 3 mL of HCl into your graduated cylinder B. Drop a strip of Mg into the cylinder and loosely seal the top of the cylinder with a ball of clay as you both observe… Do not plug the cylinder too tightly… C. Observe the reaction for 2 minutes - while you are observing, take the temperature in the area around the Mg with the temperature gun. D. After 2 minutes, proceed to “Reaction 2” instructions (you can come back and answer the questions below at the end of the lab) Question: What do you see that would indicate there is a chemical change occurring between the HCl and Mg in the graduated cylinder? Question: Based on the ‘rule’ we discussed about identifying chemical changes (a new substance is formed), how does this prove there is a chemical change occurring? Question: What was the temperature where the reaction is occurring between the Mg and the HCl? Question: How does this indicate that a chemical change (reaction) was occurring? REACTION #2: While one partner prepares to remove the clay stopper on the graduated cylinder, the other partner will light the lighter, then as quickly and smoothly as possible, remove the clay stopper and hold the flame to the top of the cylinder. Research: Do some quick research on the properties of hydrogen gas and record your findings below: Question: What do you see that would indicate there was a chemical reaction occurring when you ignited the hydrogen gas? List the clues below: Question: Based on what you observed during the first reaction with HCl + Mg, attempt to explain what happened in both the first reaction and the second reaction below. We know there was a chemical reaction, but do a little research on these reactions to help you explain what exactly happened. Reaction #1: Reaction #2: