File

Questions on Electrochemical cells (2) (AHL) - Electrolysis
1. Electricity was passed through two different electrolytic cells connected in series. One of the cells contained dilute sulfuric acid solution and 250 cm 3 of oxygen (measured at STP) was evolved at the positive electrode (anode). The second cell contained molten lead bromide. (I mole of gas occupies 22.7 dm 3 at STP).
(a) Deduce the volume of hydrogen that was released (measured at STP) at the negative electrode
(cathode) in the first cell.
(b) Deduce the mass of lead that was formed in the second cell.
(c) During the electrolysis the current was supposed to have been kept constant at 0.500 A but in fact it fluctuated to give an average reading of 0.450 A. Describe how this would affect the answer to part (b).
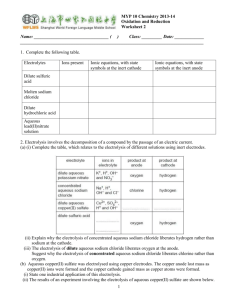
2. (a) Explain how substituting copper electrodes in place of graphite electrodes affects the products produced during the electrolysis of aqueous copper(II) sulfate solution.
(b) Describe and explain what will happen to the colour of the solution in the above two cases.
A____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
B____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
3. Describe how you could cover a piece of steel with a thin layer of silver using a solution of silver nitrate.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
4. State the relevant half-equations to explain what happens at the two electrodes when electricity is passed through (a) a very dilute aqueous solution of sodium chloride and (b) a much more concentrated aqueous solution of sodium chloride.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
5. Explain why both the electrolysis of dilute aqueous sodium hydroxide solution and the electrolysis of dilute aqueous sulfuric acid are both sometimes known as the electrolysis of ‘water’.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
6. Predict what will be formed at (a) the negative electrode (cathode) and (b) the positive electrode
(anode) when electricity is passed through a dilute aqueous solution of nickel(II) chloride.
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
_____________________________________________________________________________________
Answers
1. (a) 500 cm 3 . Since 4 mol of electrons are required to form one mol of oxygen and only 2 mol of electrons are required to form 1 mol of hydrogen twice the amount (and hence twice the volume) of hydrogen will be produced.
(b) 2H + (aq) + 2e – → H
2
(g) and Pb 2+ (l) + 2e – → Pb(l) so the same amount of lead (in mol) will be formed as hydrogen produced.
Amount of hydrogen = 250/22700 = 0.0110 mol; A r
for Pb = 207.20
Mass of lead = 0.0110 x 207.20 = 2.28 g
(c) It would have no effect. The cells were connected in series so the same quantity of electricity passed through both cells.
2. (a) With graphite electrodes copper is deposited at the (–) electrode: Cu 2+ (aq) + 2e – →Cu(s) and oxygen is evolved at the (+) electrode: 2H
2
O(l) → 4H + (aq) + O
2
(g) + 4e –
With copper electrodes, copper is still deposited at the (–) electrode but the (+) electrode reacts to produce copper ions and electrons so no gas is evolved: Cu(s) → Cu 2+ (aq) + 2e –
(b) With graphite electrodes the intensity of the blue colour decreases and the solution eventually goes colourless as all the aqueous copper ions (which cause the blue colour) are deposited as copper. With copper electrodes the intensity of the blue colour remains constant as each copper ion that is deposited as copper metal is replaced by a copper ion going into solution from the positive electrode.
3. Make the piece of steel the negative electrode and ideally make the positive electrode from a piece of pure silver. During the electrolysis the silver ions will be reduced (and deposited as silver on the steel electrode) in preference to hydrogen ions from the water as they are above hydrogen in the electrochemical series. The equation is: Ag + (aq) + e – → Ag(s)
4. (a) (–) electrode (cathode): 2H
2
O(l) + 2e – → H
2
(g) + 2OH – (aq) (or 2H + (aq) + 2e – → H
2
(g) )
(+) electrode (anode): 2H
2
O(l) → 4H + (aq) + O
2
(g) + 4e –
(b) (–) electrode (cathode): 2H
2
O(l) + 2e – → H
2
(g) + 2OH – (aq) (or 2H + (aq) + 2e – → H
2
(g) )
(+) electrode (anode): 2Cl – (aq) → Cl
2
(g) + 2e – (mainly - although some oxygen will also still be evolved.)
5. Both give two volumes of hydrogen, formed at the negative electrode (cathode), to every one volume of oxygen, formed at the positive electrode (anode). i.e. H
2
O. Pure water itself is a poor conductor of electricity but the ions in the NaOH(aq) or H
2
SO
4
(aq) cause the 'water' to conduct.
6. (a) (–) electrode (cathode): H
2
(g) evolved as H + (aq) ions are more readily reduced than Ni 2+ (aq).
(b) (+) electrode (anode): O
2
(g) evolved as it is formed in preference to Cl
2
(g) in dilute solutions of Cl –
(aq).