Design, synthesis and evaluation of 3,4
advertisement

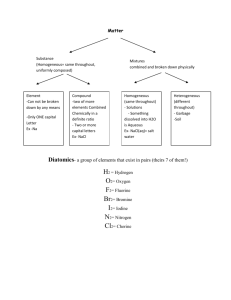
Design, synthesis and evaluation of 3,4-dihydroxybenzoic acid derivatives as antioxidants, bio-metal chelating agents and acetylcholinesterase inhibitors. Laura Friggeri a, Daniela De Vita a*, Fabiana Pandolfi a, Silvano Tortorella a, Roberta Costi a, Roberto Di Santo a and Luigi Scipione a Complexation studies pag.2 Enzymatic inhibition studies pag.8 Molecular docking studies pag.10 1 Complexation studies UV spectra and Job’s plot obtained for each compound with metal cations Cu2+, Fe2+, Fe3+ are shown in figures S1-S12 in the next pages. Fig- S1 compound 8 in presence of Cu2+ Fig- S2 compound 8 in presence of Fe2+ 2 Fig- S3 compound 8 in presence of Fe3+ Fig- S4 compound 9 in presence of Cu2+ 3 Fig- S5 compound 9 in presence of Fe2+ Fig- S6 compound 9 in presence of Fe3+ 4 Fig- S7 compound 10 in presence of Cu2+ Fig- S8 compound 10 in presence of Fe2+ 5 Fig- S9 compound 10 in presence of Fe3+ Fig- S10 compound 11 in presence of Cu2+ 6 Fig- S11 compound 11 in presence of Fe2+ Fig- S12 compound 11 in presence of Fe3+ 7 Enzymatic inhibition studies Dixon’s plot obtained for all compounds with Electric eel AChE are presented in the figures S13 – S16 in the next pages. 0,0070 0,0065 0,0060 0,0055 1/Rate (µmol/min) 0,0050 0,0045 0,0040 0,0035 0,0030 0,0025 0,0020 ASCh = 200 M ASCh = 300 M ASCh = 400 M 0,0015 0,0010 0,0005 -6 -5 -4 -3 -2 -1 0 1 2 3 4 [8] (µM) Fig- S13. Dixon plot of obtained for AChE in presence of increasing concentrations of carbamate 8. Noncompetitive inhibition was observed (Ki = 5.8 ± 0.4 μM). 0,012 0,011 0,010 1/Rate (µmol/min) 0,009 0,008 0,007 0,006 0,005 0,004 ASCh = 200M ASCh = 300M ASCh = 400M 0,003 0,002 0,001 -4 -3 -2 -1 0 1 2 3 4 [9] (µM) Fig- S14.. Dixon plot of obtained for AChE in presence of increasing concentrations of carbamate 9. Mixed inhibition was observed (Ki = 8.5 ± 5.6 μM). 8 0,012 0,011 0,010 1/Rate (µmol/min) 0,009 0,008 0,007 0,006 0,005 0,004 ASCh = 200M ASCh = 300M ASCh = 400M 0,003 0,002 0,001 -6 -5 -4 -3 -2 -1 0 1 2 3 4 [10] (µM) Fig- S15.. Dixon plot of obtained for AChE in presence of increasing concentrations of carbamate 10. Mixed inhibition was observed (Ki = 18.9 ± 17.0 μM). 0,010 0,009 0,008 1/Rate (µmol/min) 0,007 0,006 0,005 0,004 ASCh = 400 M ASCh = 500 M ASCh = 600 M 0,003 0,002 0,001 -15 -12 -9 -6 -3 0 3 6 9 12 15 [11] (µM) Fig- S16. Dixon plot of obtained for AChE in presence of increasing concentrations of carbamate 11. Mixed inhibition was observed (Ki = 1.5 ± 1.1 μM). 9 Molecular docking studies The best binding pose identified by EADock DSS software vs native binding mode of donepezil in the crystal structure of TcAChE (1EVE) is reported in figure S17. The best binding poses of carbamates 8-11 in the same protein are reported in figures S18-S21. Fig- S17. The superposition of the re-docked Donepezil (orange) and the native binding mode of Donepezil (pink) into the active site of TcAChE (1EVE). 10 Fig- S18 The best binding pose of the compound 8 (yellow) in the active site of TcAChE (1EVE). The selected aminoacids are dark cyan colored. Fig- S19 The best binding pose of the compound 9 (blue) in the active site of TcAChE (1EVE). The selected aminoacids are dark cyan colored. 11 Fig- S20 The best binding pose of the compound 10 (red) in the active site of TcAChE (1EVE). The selected aminoacids are dark cyan colored. Fig- S21 The best binding pose of the compound 11 (green) in the active site of TcAChE (1EVE). The selected aminoacids are dark cyan colored. 12 The best binding poses of carbamates 8-11 identified by EADock DSS software in the crystal structure of hBChE (1P0I) are reported in figures S22-S25. Fig- S22 The best binding pose of the compound 8 (magenta) in the active site of hBChE (1P0I). The selected aminoacids are orange colored. 13 Fig- S23 The best binding pose of the compound 9 (magenta) in the active site of hBChE (1P0I). The selected aminoacids are orange colored. 14 Fig- S24 The best binding pose of the compound 10 (blue) in the active site of hBChE (1P0I). The selected aminoacids are orange colored. 15 Fig- S25 The best binding pose of the compound 11 (magenta) in the active site of hBChE (1P0I). The selected aminoacids are orange colored. 16