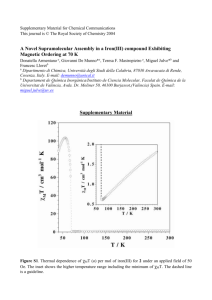
Analysis for determination of binding constant
advertisement

© The Royal Society of Chemistry 2000 Supplementary Data A dibenzo-16-crown-5 fluoroionophore for selective emission ratio sensing of Na+ in basic aqueous dioxane solution Takashi Hayashita,*a Shinichiro Taniguchi,a Yoshihiko Tanamura,a Tatsuya Uchida,a Seiichi Nishizawa,a Norio Teramae,*a Yong Suk Jin, b Jong Chan Lee,b and Richard A. Bartschc a Department of Chemistry, Graduate School of Science, Tohoku University, Aoba-ku, Sendai 980-8578, Japan b c Department of Chemistry, Chung-Ang University, Seoul 156-756, Korea Department of Chemistry and Biochemistry, Texas Tech University, Lubbock, Texas 79409-1061, USA © The Royal Society of Chemistry 2000 Determination of binding constant: The 1:1 binding constant of 1 for Na+ is calculated from a Benesi-Hildebrand plot analysis.a On the assumption that the fluorescence change is only induced by the formation of a 1:1 complex between 1 (L) and metal ion (M), the fluorescence intensity (I) can be expressed by the following equations: I = L[L] + ML[ML] (A-1) [Lt] = [L] + [ML] (A-2) [ML] K= (A-3) [M][L] where L and ML are the fluorescence quantum yields for 1, and denotes the instrumental constant including the excitation light intensity and the molar absorptivity of 1. From these equations, the Benesi-Hildebrand type equation (A-4) can be derived as, 1 I = 1 + bDfK[L t][ M] 1 bDf[L t] DI = I - I 0, Df = f ML - f L (A-4) (A-5) The double-reciprocal plots of fluorescence intensity recorded at 387 nm and 459 nm as a function of Na+ concentration according to equation (A-4) are shown in Fig. A. All plots show linear relationship with correlation coefficients of higher than 0.995, and the 1:1 binding constant is obtained from the following equation, K = (y-intercept)/(slope) (A-6) We have repeated this experiment twice under the same experimental condition, and obtained the average binding constant value of 21917 (M-1). Reference (a) K. A. Connors, Binding Constants; Wiley, New York, 1987, pp. 147-187. © The Royal Society of Chemistry 2000 -1 -1 R = 0.997 K = 202 M -1 -2 1/ D Iat 387 nm -2 (a) 387 nm -3 -3 -4 -4 50 0 100 200 300 400 (b) 459 nm 40 R = 0.996 K = 205 M -1 1/ D Iat 459 nm 40 30 30 20 20 0 10 10 0 100 100 200 200 300 300 400 400 [Na +]-1 / M-1 Fig. A Benes i-Hildebrand plots at (a) 387 nm and (b) 459 nm. In 4:1 1,4-dioxane-water (v/v), [ 1] = 8.0x10 [TMAOH] = 33 mM. ex = 355 nm. -7 M,