name: Hiep Ton
advertisement

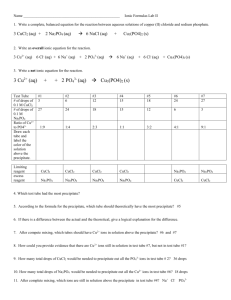
NAME: HIEP TON October 20, 2014 LAB 4 PRECIPITATION REACTION I. OBJECTIVE : • To observe the precipitation formed. • To analyze and determine the limit reagent. • To understand how important limit reagent might affect the amount of precipitate. II. DATA and REPORT : 1 – INDIVIDUAL WORK: A. Dissolve about 1 cm3 of cobalt nitrate (s), Co(NO3)2, in about 20 mL of distilled water. Describe the appearance of solution. Observation: The solution turns red as the Cobalt Nitrate dissolved totally in water. B. Dissolve about 1 cm3 of sodium phosphate (s), Na3PO4, in about 20 mL of distilled water. Describe the appearance of solution. Observation: The Sodium Phosphate seems not to dissolve totally in the water as it remains some solid in the solution. The color is colorless. C. Pour half of each A and B solutions into a third beaker and mix thoroughly. (Save the mixture and the remaining solutions of A and B for later use.) Describe the appearance of the mixture. Observation: The solution turns purple immediately as the precipitate is formed. D. Assuming that the reaction involves the coming together of dissolved ions what are the possible identities of the solid formed in the mixture in C? Write a balanced chemical equation for this reaction. Answer: 2 Na3PO4 (aq) + 3 Co(NO3)2 (aq) → Co3(PO4)2 (s) + 6 NaNO3 (aq) E. Design an experiment using the chemicals provided by the instructor that would prove to give the same precipitate. Describe the results of these experiments. Instructed to skip. Did not do part E. F. Separate the mixture by filtration. Note the characteristics of the liquid, called the supernatant, and predict all of the materials which might be dissolved in the liquid. Observation: Cobalt Nitrate is totally gone since the remain supernatant is colorless. Page 1 of 6 NAME: HIEP TON October 20, 2014 G. Divide the supernatant in half and test each half with the remaining Co(NO3)2 and Na3PO4 solutions. Describe the results: Observation: Adding Cobalt Nitrate, the solution forms precipitate again. Adding Sodium Phosphate, nothing happened. H. What conclusions can be drawn from these data concerning the chemicals present in the supernatant? Is one reactant in excess? (How might changing the original amounts of Co(NO3)2 and Na3PO4 affect the composition of the supernatant?) Answer: From the result of part G, then the amount of Cobalt Nitrate is the limit reagent since it is the first solution that was totally dissolved. 2 – GROUP WORK: Date Table 1: Initial Buret reading Na3PO4 Final Buret reading Na3PO4 Volume (mL) of Na3PO4 Group #: B . Na3PO4: 10.0 mL – 0.0950M Co(NO3)2: 20.0 mL – 0.100M 0.10 mL 10.10 mL 10.00 mL Initial Buret reading Co(NO3)2 Final Buret reading Co(NO3)2 Volume (mL) of Co(NO3)2 1.02 mL 21.20 mL 20.18 mL Mass (g) of Paper Mass (g) of Paper & PPT Mass (g) of PPT 1.3062 g 1.6069 g 0.3007 g A. Describe the appearance of each of the supernatant solutions produced in B. Answer: The color was purple pink B. Divide each of the supernatants into two parts. Test one part with a dropperful of Co(NO3)2 and the other with a dropper full of Na3PO4 solution. Summarize the results of these tests below. Answer: Cobalt Nitrate – nothing happened, remained the same Sodium Phosphate – formed precipitate and turned color as purple C. Explain the results of this testing of the supernatants. What do these results show Page 2 of 6 NAME: HIEP TON October 20, 2014 must have happened in each reaction mixture? Why can you form more precipitate from a supernatant? Why don’t all three supernatants give the same test results? Could you have predicted these test results from your balanced equation and masses of salts added? Answer: The supernatant reacted with Sodium Phosphate and continued to forme precipitate therefore Sodium Phosphate is the limited reagent. Since the Sodium Phosphate was not enough when mixed with Cobalt Nitrate, the supernatant was still being able to react with the added Sodium Phosphate. D. After the washings, put the filter papers with their filtrates on labeled paper towels (your group name and date should be written on this paper towel.) and then in a 100 oC drying oven. Keep the samples for a week or until the next laboratory period. Answer: 1. Firs time weighting, did not put the filter papers on a 100oC drying oven. And the time was from Monday to Wednesday (October 13th, 2014 – October 15th, 2014) and the mass of paper and ppt was measured at 3.3206 gram. 2. Second time weighting, did not put the filter papers on a 100oC drying oven. And the time was from Monday to Wednesday (October 13th, 2014 – October 20th, 2014) and the mass of paper and ppt was measured at 1.6069 gram. E. Your data will be graphed with data from the rest of the class. Your instructor has set up a computer where you may include your results. Enter the mass of sodium phosphate for each solution on the x-axis and the mass of the precipitate for each solution on the y-axis. Data Table of Volume Na3PO4 (mL) & Mass of Precipitate (g): Group A B C D E F G H I Volume Na3PO4 (mL) Mass of Precipitate (g) 2.0 0.0835 10.0 0.3007 22.0 0.1905 4.0 0.1271 12.0 0.3052 20.0 0.3359 8.0 0.2032 16.0 0.4093 24.0 0.3351 Page 3 of 6 NAME: HIEP TON October 20, 2014 Data Table of Volume Na3PO4 (mL) & Mass of Precipitate (g): 0.45 0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 5 10 15 20 25 30 x-axis: mass of sodium phosphate for each solution. y-axis: the mass of the precipitate for each solution. III. DATA and CALCULATION : Na3PO4: 10.0 mL (or 0.0100L) – 0.0950M Co(NO3)2: 20.0 mL (or 0.0200L) – 0.100M Balanced Equation: 2 Na3PO4 (aq) + 3 Co(NO3)2 (aq) → Co3(PO4)2 (s) + 6 NaNO3 (aq) Mole of Na3PO4: n = M x V = 0.0950M x 0.0100L = 9.5 x 10-04 mol Mass (g) of Na3PO4: (22.990 ×3)+(30.974)+(15.999 ×4)𝑔𝑟𝑎𝑚 9.5 x 10-04 mol × = 0.155743 gram 1 𝑚𝑜𝑙 Mole of Co(NO3)2: n = M x V = 0.100M x 0.0200L = 2 x 10-03 mol Mass (g) of Co(NO3)2: (58.933)+(14.007 ×2)+(15.999 ×6)𝑔𝑟𝑎𝑚 2 x 10-03 mol × = 0.365882 gram 1 𝑚𝑜𝑙 Molar mass of Co3(PO4)2: Co3(PO4)2 = (58.933 x 3) + (30.974 x 2) + (15.999 x 8) = 366.739 gram/1mol Page 4 of 6 NAME: HIEP TON October 20, 2014 Theoretical mass (g) of Co3(PO4)2: 1. Na3PO4: 9.5 x 10-04 mol Na3PO4 × 1mol Co3(PO4)2 2 mol Na3PO4 × 366.739 gram Co3(PO4)2 1mol Co3(PO4)2 = 0.1742gram 2. Co(NO3)2: 2 x 10-03 mol × 1mol Co3(PO4)2 3 mol Na3PO4 × 366.739 gram Co3(PO4)2 1mol Co3(PO4)2 = 0.2445gram Since the gram that Co3(PO4)2 could be produced from Na3PO4 is lesser than Co(NO3)2 could, Na3PO4 was the limit reagent. And the mass of Co3(PO4)2 produced from Na3PO4 can be used to calculate the percent yield. Percent yield: mass of obtained theorectical mass × 100% = 0.3007 gram 0.1742gram × 100% = 172.62% Summary Calculation Table: Mass (g) of Na3PO4 Mass (g) of Co(NO3)2 Theoretical mass (g) of Co3(PO4)2 from Na3PO4 Theoretical mass (g) of Co3(PO4)2 from Co(NO3)2 Percent yield IV. 0.155743 gram 0.365882 gram 0.1742gram 0.2445gram 172.62% DISCUSSION : Without following the procedure instruction precisely, the measurement of weighting came out to be unreasonable as the first time weighting of the mass of paper and ppt was still contained partially some amount of water. Even though the mass of paper and ppt was dramatically dropped from the first one, the result of percent yield was unexpected as it excessed up to 172.62%. The error might cause by not following the instruction from procedure that the paper and ppt should have been placed on a 100oC drying oven. And the unexpected result of the mass obtained comparing to the theoretical mass might be caused by the imprecise measuring process of the amount of the solutions Page 5 of 6 NAME: HIEP TON October 20, 2014 from the burets as well as weighting process might be effected by the surrounding environment with a very small factor that was enough to cause the change in the result. V. CONCLUSION: Being able to analyze and determine the limit reagent from the experiments through observation and theoretical balanced equation. As using the remained supernatant to test in the experiments, the result of the amount of precipitate depends totally on the limit reagent as it holds an important factor of the mass of outcome precipitate. The limit reagent is various and depending on the amount of the solutions when become a mixture. The concept of limit reagent does not limit to how much the volume of a certain solutions but which solution has actually gone out before another while forming the precipitation. Page 6 of 6