Name Ionic Formulas Lab II 1. Write a complete, balanced equation
advertisement
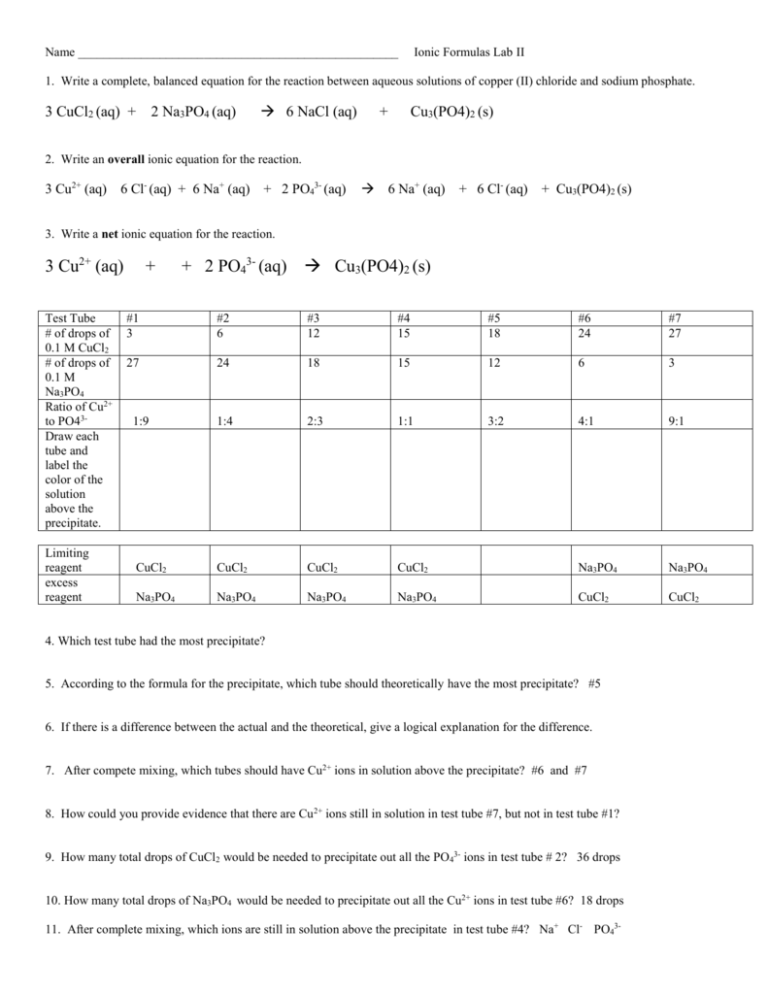
Name ___________________________________________________ Ionic Formulas Lab II 1. Write a complete, balanced equation for the reaction between aqueous solutions of copper (II) chloride and sodium phosphate. 3 CuCl2 (aq) + 2 Na3PO4 (aq) 6 NaCl (aq) + Cu3(PO4)2 (s) 2. Write an overall ionic equation for the reaction. 3 Cu2+ (aq) 6 Cl- (aq) + 6 Na+ (aq) + 2 PO43- (aq) 6 Na+ (aq) + 6 Cl- (aq) + Cu3(PO4)2 (s) 3. Write a net ionic equation for the reaction. 3 Cu2+ (aq) Test Tube # of drops of 0.1 M CuCl2 # of drops of 0.1 M Na3PO4 Ratio of Cu2+ to PO43Draw each tube and label the color of the solution above the precipitate. Limiting reagent excess reagent + + 2 PO43- (aq) Cu3(PO4)2 (s) #1 3 #2 6 #3 12 #4 15 #5 18 #6 24 #7 27 27 24 18 15 12 6 3 1:9 1:4 2:3 1:1 3:2 4:1 9:1 CuCl2 CuCl2 CuCl2 CuCl2 Na3PO4 Na3PO4 Na3PO4 Na3PO4 Na3PO4 Na3PO4 CuCl2 CuCl2 4. Which test tube had the most precipitate? 5. According to the formula for the precipitate, which tube should theoretically have the most precipitate? #5 6. If there is a difference between the actual and the theoretical, give a logical explanation for the difference. 7. After compete mixing, which tubes should have Cu2+ ions in solution above the precipitate? #6 and #7 8. How could you provide evidence that there are Cu2+ ions still in solution in test tube #7, but not in test tube #1? 9. How many total drops of CuCl2 would be needed to precipitate out all the PO43- ions in test tube # 2? 36 drops 10. How many total drops of Na3PO4 would be needed to precipitate out all the Cu2+ ions in test tube #6? 18 drops 11. After complete mixing, which ions are still in solution above the precipitate in test tube #4? Na+ Cl- PO43-