Precipitation Reaction - Experiment 7 (Part I)

Precipitation Reaction - Experiment 6 (Part I)
I. PRE-LAB
A. What is a precipitate?
A precipitate is an insoluble ionic compound that is formed in solution.
B. How might a limiting reagent effect the amount of precipitate that is formed?
A limiting reagent will determine how much precipitate can be formed.
II. PROBLEM STATEMENTS
What is the reaction between Co(NO
3
)
2
and Na
3
PO
4
? Are all of the reactants consumed?
Why or why not?
III. QUALITATIVITE PROPERTIES OF COBALT NITRATE AND SODIUM
PHOSPHATE INTERACTIONS
A. Dissolve about 1 cm
3
of cobalt nitrate (s), Co(NO
3
)
2
, in about 20 mL of distilled water. Describe the appearance of solution.
Students should describe color.
B. Dissolve about 1 cm 3 of sodium phosphate (s), Na
3
PO
4
, in about 20 mL of distilled water. Describe the appearance of solution.
Students should describe color.
C. Pour half of each A and B solutions into a third beaker and mix thoroughly.
(Save the mixture and the remaining solutions of A and B for later use.)
Describe the appearance of the mixture.
This will vary slightly but all students should obtain a purple precipitate.
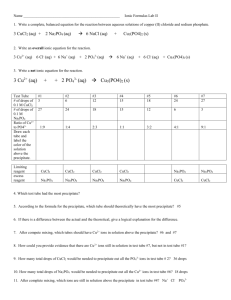
D. Assuming that the reaction involves the coming together of dissolved ions what are the possible identities of the solid formed in the mixture in C? Write a balanced chemical equation for this reaction.
3Co(NO
3
)
2
(aq) + 2Na
3
PO
4
(aq)
Co
3
(PO
4
)
2
(s) + 6NaNO
3
(aq)
3Co +2 (aq) + 6NO
3
-1 (aq) + 6Na + (aq) + 2PO
4
+3 (aq)
39
3Co +2 (aq) + 6NO
3
-1 (aq) + 6Na + (aq) + 2PO
4
+3 (aq)
Students may wish to consult their solubility table if they have reached this topic in lecture.
E.
Design an experiment using the chemicals provided by the instructor that would prove give the same precipitate. Describe the results of these experiments.
There are several possibilities that the students can come up with.
Here is an example:
3CoCl
2
(aq) + 2K
3
PO
4
(aq)
Co
3
(PO
4
)
2
(s) + 6KCl (aq)
In this case they must recognize that the purple precipitate could be Co
3
(PO
4
)
2
.
Students may also wish to again use their solubility table and not the chemicals provided. This is acceptable, but they must indicate that they are using the solubility table.
F. Separate the mixture by filtration. Note the characteristics of the liquid, called the supernatant, and predict all of the materials (ions etc.) which might be dissolved in the liquid.
The instructor may need to point out to the students that this is the mixture in IIIC.
The instructor should also show students how to fold a filter paper. It is not necessary to flute the paper since quartering the paper works well.
Make certain that students indicate the color of the spernatant
The ions present are Co +2 ,NO
3
-1 ,Na + ,PO
4
+3 . Make certain that the ions contain the correct charges. Some students at this point may not realize that excess cobalt or phosphate can pass into the supernatant. This is acceptable as long as they come to the correct conclusions in the next question.
While the mixture is filtering students may wish to get started on PART IV. In order to do this they must have been assigned a set of solutions at the beginning of class (see the the INSTRUCTOR’S NOTES in PART IV).
G . Divide the supernatant in half and test each half with the remaining Co(NO
3
)
2
and
Na
3
PO
4
solutions. Describe the results:
Some students will have excess phosphate in solution (indicated by the addition of
Co(NO
3
)
2
) and some students may have excess cobalt in solution (indicated by the pink color of the supernatant and the addition of Na
3
PO
4
) .
40
On occasion, some students will not obtain any additional precipitate from the supernatant. In this case it is important that students realize that they initially added the two solutions together in the correct stoichiometric proportions.
H. What conclusions can be drawn from these data concerning the chemicals present in the supernatant? Is one reactant in excess? (How might changing the original amounts of
Co(NO
3
)
2
and Na
3
PO
4
affect the composition of the supernatant?)
Students should give a detailed explanation of what they observed in IIIG. They should also be able to realize that altering the proportions of the different starting materials may change their results when attempting to obtain more precipitate from the supernatant.
IV.
QUNTITATIVE PROPERTIES OF COBALT NITRATE AND SODIUM
PHOSPHATE INTERAACTIONS
***** INSTRUCTOR’S NOTES:
Divide the class so that each group of students must prepare either solutions ABC,
DEF, or GHI. With a class of 28 there should be at least 2 groups for each set of solutions. It is important to follow this format so that each group of students will have a set of solutions that will give them very different amounts of precipitate.
At the Instructors lab bench set up four burets; two containing sodium phosphate and two containing cobalt nitrate. It may be necessary to demonstrate how to dispense the solutions. The instructor will also have to refill the burets during the lab session (DO
NOT ALLOW THE STUDENTS TO REFILL THE BURETS).
The students will be dispensing varying amounts of solution from each buret. It is not important that the students dispense the exact amount of solution indicated, but it is important that the students record both the initial and final buret readings in the table provided on page 43. The collective class data obtained from this part of the experiment will be used to make a graph in Experiment 6 PART II. This graph must be done during the next lab session after students have collected and oven dried their precipitates. Directions for setting up the graph are provided in Experiment 6 PART
II.
Before students begin filtering their solutions they must weigh and label all three filter papers. The labels should contain the names of the students as well as indicate which precipitate will be collected in the filter paper (A, B C, …). The mass of the filter papers should be recorded on page 43.
A.
Your instructor will assign three solutions for your group to prepare.
41
Solutions Na
3
PO
4
mL Co(NO
3
)
2
mL
A 2.0 20.0
B
C
10.0
22.0
20.0
20.0
D
E
F
G
4.0
12.0
20.0
8.0
20.0
20.0
20.0
20.0
H
I
16.0
24.0
20.0
20.0
Prepare three clean 100 mL beakers and label them according to the solutions you have been assigned. In each beaker dispense 20.0 mL of Co(NO
3
)
2
solution from the buret provided by the instructor. Make sure that you record the initial and final reading on the buret in the table provided in Part 2 of Experiment 7 as well as the concentration of the solution.
Into each beaker containing 20.0 mL of Co(NO
3
)
2
measure the appropriate amount of
Na
3
PO
4
, e.g. If you have been assigned Solution A, it should contain 20.0 mL Co(NO
3
)
2 and 2.0 mL of Na
3
PO
4
. The Na
3
PO
4
solution should also be dispensed using the burets provided by the instructor. Make sure that you record the concentration of the solution as well as the initial and final readings of the buret in the data table. Mix each solution thoroughly and allow it to stand for at least 10 minutes. Calculate the masses of salts added to each beaker and record the amounts in the data table.
B.
Label and weigh three sheets of filter paper, recording their masses in the table. Mix and filter each of the three reaction mixtures. Catch the first 15-20 mL of supernatant from each filtration in separate, clean and labeled beakers. Set these aside for later analysis below.
C. Continue the filtration into other beakers. Then wash each, precipitate with two 5 mL portions of water. Use these water washes to transfer any precipitate remaining in the original beakers to the filter paper.
1.
Describe the appearance of each of the supernatant solutions produced in B.
Descriptions may vary but at least one of the solutions should be pink and at least one should be clear.
2. Divide each of the supernatants into two parts. Test one part with a dropperful of
Co(NO
3
)
2
and the other with a dropper full of Na
3
PO
4
solution. Summarize the results of these tests below.
The students will have three supernatants. Each of which will be divided in two and tested with additional cobalt nitrate and additional sodium phosphate.
42
One of the supernatants should be pink and have excess cobalt ion. Upon addition of sodium phosphate more purple precipitate should form. Another solution should be clear and contain excess phosphate ion. This will be indicated when more precipitate is formed by the addition of cobalt nitrate. The third solution may have varying results depending on how carefully the students dispensed each of the starting materials and on which solutions they have been assigned. The mass and number of moles for each starting material as well as the theoretical yields have been calculated and can be found in Experiment 6 PART II.
3.
Explain the results of this testing of the supernatants. What do these results show must have happened in each reaction mixture? Why can you form more precipitate from a supernatant? Why don’t all three supernatants give the same test results? Could you have predicted these test results from your balanced equation and masses of salts added?
For each solution, students should indicate which starting material must have been in excess in order to obtain more precipitate from the supernatant.
D.
After the washings, put the filter papers with their filtrates on labeled paper towels
(your group name and date should be written on this paper towel.) and then in a 100 o
C drying oven. Keep the samples for a week or until the next laboratory period.
43
C
D
E
F
G
Precipitation Reaction - Experiment 6 (Part II)
***** INSTRUCTOR’S NOTES:
A
Set up the G3 Macintosh for overhead projection. Open the LoggerPro file entitled
Experiment 6-INST. This file is found in the Chemistry 101 Folder. After students have weighed their dried precipitates they will the graph their data. They should enter the mass of the dried precipitate obtained from each solution as well as the mass of sodium phosphate that was initially used. Students will have to calculate the mass of sodium phosphate based on the concentration of the solution. The mass, number of moles and theoretical yields are as follows:
Sol Na
3
PO
4 mL
2.0
Na
3
PO moles
4
0.00019*
Na
3
PO grams
0.031
4
Co(NO mL
20.0
3
)
2
Co(NO
3 moles
)
0.00200
2
Co(NO
0.366
3 grams
)
2
Co
3
(PO
4
)
Theoret.
Yield (g)
0.035
2
B 10.0 0.00095* 0.16 20.0 0.00200 0.366 0.17
H
I
22.0
4.0
12.0
20.0
8.0
16.0
24.0
0.0021
0.00038*
0.0011*
0.0019
0.00076*
0.0015
0.0023
0.34
0.062
0.19
0.31
0.12
0.25
0.37
20.0
20.0
20.0
20.0
20.0
20.0
20.0
0.00200*
0.00200
0.00200
0.00200*
0.00200
0.00200*
0.00200*
0.366
0.366
0.366
0.366
0.366
0.366
0.366
0.24
0.070
0.20
0.24
0.14
0.24
0.24
* Indicates Limiting Reagent
44
The graph should be very similar to the example shown below:
45
I. Take your samples out from the drying oven and let it cool for 5 minutes. Then weigh them.
Record the data in the table. Get three other sets of data from other students. Make sure you choose other students’ data with a different amount of Na
3
PO
4
.
Concentration of stock Na
3
PO
4
solution 0.0156 g / mL (0.0950M)
Concentration of stock Co(NO
3
)
2
0.0183 g / mL (0.100 M)
Initial buret reading
Na
3
PO
4
Final buret reading
Na
3
PO
4
Solution: A
Volume (mL) of Na
3
PO
4
2.0 mL
Solution:B
10.0 mL
Solution: C
22.0 mL
Mass (g) of Na
3
PO
4
0.031 0.16
20.0 mL
This must be calculated by the students
Initial buret reading
Co(NO
3
)
2
Final buret reading
Co(NO
3
)
2
Volume(mL) of Co(NO
3
)
2
20.0 mL
Mass (g) of Co(NO
3
)
2
0.366 0.366
This must be calculated by the students
Mass (g) of Paper
Mass (g) of PPT
Weighed prior to filtration
Mass (g) of Paper & PPT
Weighed after oven drying.
0.24
0.366
20.0 mL
46
II. Your data will be graphed with data from the rest of the class. Your instructor has set up a computer where you may include your results. Enter the mass of sodium phosphate for each solution on the x-axis and the mass of the precipitate for each solution on the y-axis.
A. Attach a copy of the graph to the facing page.
See sample on previous page.
B.
Notice that regression analysis is not appropriate for this curve. Why? Explain each section of the graph as well as the quality of the data obtained. Make sure to discuss limiting reagents in your analysis.
Regression analysis is not appropriate because the graph is not linear. The student should indicate that the sodium phosphate is the limiting reagent in the first part of the graph while the cobalt nitrate is the limiting reagent in the second part of the graph.
47