Quantum Numbers - Solon City Schools
advertisement

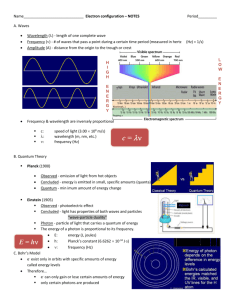
Honors Chemistry Unit 3 Quantum numbers Electron orbital shapes Rules: o Aufbau principle o Hund’s Rule o Pauli Exclusion principle Orbital notations Electron configuration Noble gas notation 1 We are learning to: 1. Describe the quantum mechanical model. 2. Apply the quantum mechanical model. We are looking for: 1a. Electrons can move from ground state to an excited state by gaining specific amounts of energy (quantum) and return to the ground state by releasing a specific amount of energy (quantum) in the form of photons. 1b. Define each of the 4 quantum numbers (principal, angular momentum, magnetic, spin). 1c. Describe the shape of the various orbital shapes (s, p, d, f). 2a. Use the Aufbau principle, Hund’s rule, and Pauli exclusion to assign electron/orbital/Noble gas configurations for a given element. 2b. Identify the exceptions to the Aufbau principle and Hund’s rule for electron configurations. 2c.Give the 4 digit quantum number for a specific electron in an orbital notation. 2d. Indicate the specific electron in an orbital notation that is described by the given 4 digit quantum number. 2e. Identify the s, p, d, and f blocks on the periodic table. 2 Name _______________________________ Class _______ Flame Test: Nichrome Wire Prelab: Complete the following on a separate sheet of paper: 1. How much of each solution containing a metal ion should you transfer to your test tube? 2. What should you do to the nichrome wire prior to dipping it in a solution containing the metal ion? 3. Which metals will be tested? 4. Create a neatly drawn data table with the following 2 column headings: metal ion and flame color. 5. Create a second neatly drawn data table with the following 3 column headings: unknown, flame color, and metal ion(s) present. Purpose: 1. To observe the different colors emitted by ions in a flame test. 2. Use observations to identify the metal ion(s) in an unknown sample. Materials: Solutions containing the following metal ions: Ba2= Ca2+ Li+ K+ Test Tubes Nichrome Wire Test Tube Rack Bunsen Burner Na+ Sr2+ Cu2+ Matches/Lighter Nitric Acid **Caution! Handle very carefully! Procedure: 1. Obtain a sample of your assigned solutions from the stock solutions in the flasks up front. You only need enough of the solution to cover the loop at the tip of the nichrome wire when it is dipped into the solution. 2. Light the Bunsen burner. 3. Place tip of nichrome wire in nitric acid. 4. Place tip of nichrome wire in flame until there is a constant orange/yellow color. 5. Dip the tip of the nichrome wire into one of the solutions 6. Place the tip of the nichrome wire into the flame. 7. Observe the color of the flame as the salt/solution as it burns. 8. Repeat steps 1-5 for each salt/solution including the unknowns. Questions: (answer these on your sheet of paper with your prelab & data table) 1. Can a flame be used to identify a metal ion? Why, or why not? 2. What is happening to the electrons when we see color? 3 Schrodinger Wave Equation Only certain frequencies satisfied his mathematical equations, which described the wave properties of electrons. Heisenberg’s Uncertainty Principle: It is impossible to determine both the position and momentum of an electron at the same time, but we can give a probable location of an electron around the nucleus called an orbital. Orbital = 3D region around the nucleus that indicates the probable location of an electron To further define the orbitals (locations of electrons), quantum numbers are used. 4 Quantum Numbers Your home address is your most probable location, even though you are not there all the time. Your address is made up of four parts: state, city, street, and house number. The four quantum numbers (n, l, m, and s) can be thought of as the address for an electron in an atom. The principle quantum number, n, describes the energy level or average distance the electron is from the nucleus therefore signifying the size of the electron cloud. Although n can have numerical values from 1 to ∞, seven is the highest energy level we will use. In other words, there can be seven states, if we use our address analogy. The second quantum number, l, describes the sublevels within the energy level. These sublevels have different shapes and are represented by the letters s, p, d, f. The s sublevel is spherical in shape. The p sublevel resembles a dumbbell shape or sometimes called flower petal shape or peanut shape. The d shaped sublevel is more complicated and is described as double lobed and the f sublevel is even more complicated. There are pictures of each of these sublevels on the cover of this packet. Using our address analogy, a city is a sublevel of the state. Just as there are small states having fewer cities than large states, energy levels have different numbers of sublevels. The first energy level, n=1, is the closest to the nucleus and therefore it is the smallest electron cloud. It is so small that there is only one sublevel. The fourth energy level, n=4, is much farther away from the nucleus and therefore has more room for more sublevels. The fourth energy level has four different sublevels. The sublevels also correlate to a number as follows: s=0, p=1, d=2, f=3. These will be used when giving the four digit quantum number for a specific electron within an atom. The third quantum number, m, is the magnetic quantum number. Each sublevel contains one or more orbitals. The space occupied by one pair of electrons is called an orbital. In our address analogy, the orbitals are like the streets within a city. Just as streets are oriented north-south or east-west, orbitals are oriented in a magnetic field along the X, Y, and Z axes surrounding the nucleus of the atom. The s (spherical)sublevel contains only one orbital. The p sublevel contains three orbitals arranged along the X, Y, and Z axes. The d sublevel is made up of five orbitals, while the f sublevel contains seven orbitals. Using the address analogy, there would be seven streets in the f city. The numerical values for m range from –l to +l . Again, these will be used when giving the four digit quantum number for a specific electron within an atom. The fourth quantum number is the s or spin quantum number. It describes the clockwise or counterclockwise rotation of the electron. The numerical values for s are +½ and -½ . 5 n l m 1. Principle Quantum # (n) Energy Level 2. 3. 4. s 6 Rules/Principles to Following When Assigning Electron Locations Aufbau Principle: An electron occupies the lowest energy level available which is given by the Aufbau principle filling order. Pauli Exclusion Principle: No 2 electrons in the same atom can have the same 4 quantum numbers. Hund’s Rule: Orbitals of equal energy (and sublevel) are each occupied by one electron before any orbital is occupied by a second electron. All electrons in singularly occupied orbitals must have the same spin. Aufbau Principle Filling Order 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 4f14 5s2 5p6 5d10 5f14 6s2 6p6 6d10 6f14 7s2 7p6 7d10 7f14 7 Aufbau Principle Filling Order Applied to the Periodic Table: 8 Orbital Notation Orbital notation uses arrows up/down (for electrons) on labeled lines (orbitals). Underneath the lines (orbitals) numbers are used to represent the energy level and letters are used to show the sublevel (s, p, d, f) for the electrons. An up versus down arrow indicates the spin of the electron. When 2 electrons occupy the same orbital, they must spin in opposite directions. The orbitals are filled by following the Aufbau principle filling order and Hund’s rule with the exception of copper and chromium. Ex) orbital notation for cobalt 1s 2s 2p 3s 3p 4s _ _ _ 3d **The number of arrows should correspond to the number of electrons in the given atom of the element or electrons in the given ion of the element. Using the Aufbau filling order and Hund’s rule, write the Orbital Notation for each of the following elements on a separate sheet of paper: 1. 2. 3. 4. Carbon Argon Vanadium Chromium** 5. Antimony 6. Tungsten 7. Calcium 8. Germanium 9. Molybdenum 10. Tin 11. Copper** 12. Ba2+ Electron Configuration Electron configuration follows the same filling order as orbital notation but instead of using a line for each orbital and arrows for each electron, the number of electrons in the sublevel (s, p, d, f) is indicated by a superscript after the sublevel letter. Ex) electron configuration for cobalt 1s2 2s2 2p6 3s2 3p6 4s2 3d7 Electron Configuration practice: On a separate sheet of paper, write the full electron configuration for each of these elements. 1. 2. 3. 4. Beryllium Sulfur Scandium Copper 5. Iodine 6. Europium 7. Osmium 8. Actinium 9. Uranium 10.Seaborgium 11. O212. Mg2+ 9 Noble Gas Notation Noble gas notation is a shorthand version of electron configuration. A noble gas symbol in brackets is used to represent all the inner electrons for an atom of the given element and the configuration continues from that point on showing only the outermost electrons. Ex) Using the electron configuration for cobalt 1s2 2s2 2p6 3s2 3p6 4s2 3d7 These are the inner electrons and they would correspond to the electron configuration of argon(Ar). The Nobel gas configuration for cobalt would be [Ar] 4s2 3d7 Noble Gas Configuration practice: On a separate sheet of paper, write the full Noble gas configuration for each of these elements. 1. Aluminum 2. Iron 3. Beryllium 4. Copper 5. Gold 6. Plutonium Mixed Notation Practice Complete the following on a separate sheet of paper: 1. Write the orbital notation for the following elements: a. Nitrogen b. Chlorine c. Gallium d. Cesium 2. Write the full electron configuration for the following elements: a. Titanium b. Xenon c. Polonium 3. Write the Noble gas notation for the following elements: a. Strontium b. Iodine More Practice!!!! Complete the following on a separate sheet of paper: 1. Name each of the 4 quantum numbers along with its symbol and the information it gives for the electron. 2. Write the orbital notation for arsenic. 3. Find the 27th electron (based on filling order, including Hund’s rule) from #2 and give the four digit code for it. 4. Write the full electron configuration for Cesium. 5. Draw the electron that is represented by 4 3 +2 -1/2 10 Name __________________________________________________________________ 1. Write the orbital notation for: a. 14Si b. 29Cu 2. Write the electron configuration for: a. 16S b. 39Y 3. Write the Noble gas notation for: a. 22Ti b. 49In 4. Given the following orbital notation; predict the 4 digit quantum number that describes the circle electron: a. 1s 2s b. 1s 2s 2p 2p 4 digit quantum # _______________________ 3s 3p 4s 3d 4digit quantum # __________________________ 5. Given the 4 digit quantum number; draw /label the electron that it describes: a. 2 0 0 –½ b. 3 1 1 +½ 11 Quantum Numbers Review: 1. What is the maximum number of electrons that can be in the a. second energy level __________ b. third energy level ____________ c. fourth energy level ___________ 2. Which quantum number signifies the size of the electron cloud?__________________________ 3. The sublevel or shape of the electron cloud is designated by which quantum number?_____________________________________________________________ 4. Which quantum number is used to represent the orbital?_______________________________ 5. The s quantum number is used to describe the clockwise and counterclockwise rotation of the electron in an orbital. What two numerical values can s have?_________________________________ 6. When n has the numerical value 4, what values can l have? ____________________________ 7. When l =3, what values can m have?_______________________________________________ 8. How many orbitals are contained in each p sublevel?__________________________________ 9. How many orbitals are contained in each d sublevel?_________________________________ 10. How many electrons can be in one orbital?_________________________________________ 11. What is the maximum number of electrons that can be in a p sublevel?__________________ 12. What is the maximum number of electrons that can be in an f sublevel?__________________ 13. What is the maximum number of electrons that can be in a d sublevel?__________________ 14. What is the name of the scientist who stated that no two electrons in the same atom can have the same set of four quantum numbers?______________________________________________ 15. What is the name of the scientist who pointed out that it is impossible to know both the exact position and momentum of an electron at the same time?___________________________________ 16. What is the name of the scientist who treated the electron mathematically as a wave?______________________________________________________________________ 17. What is the name of the scientist that stated all orbitals within a sublevel must contain one electron each, all with the same spin, before two electrons will occupy the same orbital within a sublevel?___________________________________________________________________ 12