EM11_S_WS_R1(electron_configs)
advertisement

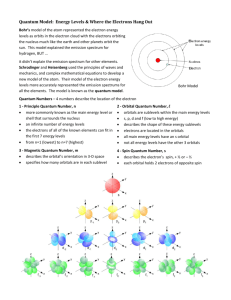
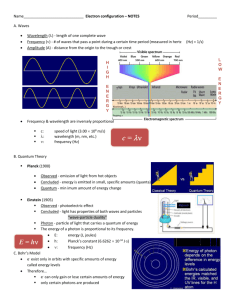
NASA-Threads Electricity and Magnetism Lesson 11: Atoms and Molecules Electron Configurations So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Most of this below is “borrowed” from Sparknotes.com. The first and most important rule to remember when attempting to determine how electrons will be arranged in the atom is Hund’s rule, which states that the most stable arrangement of electrons is that which allows the maximum number of unpaired electrons. This arrangement minimizes electron-electron repulsions. Here’s an analogy. In large families with several children, it is a luxury for each child to have his or her own room. There is far less fussing and fighting if siblings are not forced to share living quarters: the entire household experiences a lower-intensity, less-frazzled energy state. Likewise, electrons will go into available orbitals singly before beginning to pair up. All the single– occupant electrons of orbitals have parallel spins, are designated with an upward-pointing arrow, and have a magnetic spin quantum number of +1/2. As we mentioned earlier, each principal energy level, n, has n sublevels. This means the first has one sublevel, the second has two, the third has three, etc. The sublevels are named s, p, d, and f. Energy level principal quantum number, n Number of sublevels Names of sublevels 1 1 s 2 2 s, p 3 3 s, p, d 4 4 s, p, d, f At each additional sublevel, the number of available orbitals is increased by two: s = 1, p = 3, d = 5, f = 7, and as we stated above, each orbital can hold only two electrons, which must be of opposite spin. So s holds 2, p holds 6 (2 electrons times the number of orbitals, which for the p sublevel is equal to 3), d holds 10, and f holds 14. NASA-Threads Electricity and Magnetism Lesson 11: Atoms and Molecules Sublevel s p d f Number of orbitals 1 3 5 7 Maximum number of electrons 2 6 10 14 Quantum number, l 0 1 2 3 Orbital Notation Orbital notation is basically just another way of expressing the electron configuration of an atom. It is very useful in determining quantum numbers as well as electron pairing. The orbital notation for sulfur would be represented as follows: Notice that electrons 5, 6, and 7 went into their own orbitals before electrons 8, 9, and 10 entered, forcing pairings in the 2p sublevel; the same thing happens in the 3plevel. Now we can determine the set of quantum numbers. First, n = 3, since the valence electron (the outermost electron) is a 3p electron. Next, we know that p sublevels have an l value of 1. We know that ml can have a value between l and -l, and to get theml quantum number, we go back to the orbital notation for the valence electron and focus on the 3p sublevel alone. It looks like this: Simply number the blanks with a zero assigned to the center blank, with negative numbers to the left and positive to the right of the zero. The last electron was number 16 and “landed” in the first blank as a down arrow, which means its ml = -1 and ms = -1/2, since the electron is the second to be placed in the orbital and therefore must have a negative spin. So, when determining ml, just make a number line underneath the sublevel, with zero in the middle, negative numbers to the left, and positive numbers to the right. Make as many blanks as there are orbitals for a given sublevel. For assigning ms, the first electron placed in an orbital (the up arrow) gets the +1/2 and the second one (the down arrow) gets the 1/2. NASA-Threads Electricity and Magnetism Lesson 11: Atoms and Molecules Example Which element has this set of quantum numbers: n = 5, l = 1, ml = -1, and ms = -1/2? Example Complete the following table: Element Valence electron configuration Valence orbital notation Set of quantum numbers [Ar] 3d 6 5, 1, 0, +1/2 4p 5