Lab: Percent Error and Average Atomic Mass
advertisement

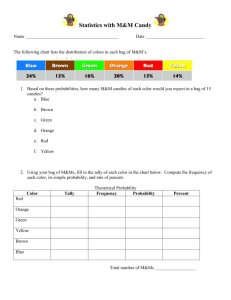
Lab: Percent Error & Average Atomic Mass Purpose: This activity is designed to introduce you to determining how much error can occur when we try to quantify chemistry with measurements. We will soon want to start answering questions such as: o o o o o -How much of a chemical is present? -What mass of a chemical is required to react? -How many liters of a chemical can react? -What is the percent error in this lab? -What is the average atomic mass for M&M’s A measurement can be compared with its accepted value by calculating its percent error. The percent error is found from the following formula: % error = Accepted value – Experimental Value x 100 Accepted Value Procedure: 1. Each person should obtain 1 bag of “isotopes” (M&M’s). 2. Analyze your individual bag contents and complete the following chart: Red Orange Yellow Green Blue Brown Total # of M&M’s How many M&M’s of each color 3. Now, combine your results with your lab partner to find the total number of each color M&M for your lab set. This is your EXPERIMENTAL DATA. Red Orange Yellow Green Blue Brown Total # of M&M’s TOTAL NUMBER of M&M’s for your lab group According to the M&M/Mars Company, M&M bags are filled according to a specific color distribution. Color blends were selected by conducting consumer preference tests, which indicate the assortment of colors that pleased the greatest number of people and created the most attractive overall effect. On average, here’s how the mix of colors is distributed: Plain M&M’s Brown…….….20.% Yellow…….….20.% Red…………….20.% Orange……….15.% Green………..15.% Blue……………10.% Each large production batch is blended precisely to those ratios and mixed thoroughly. However, since the individual packages are filled by weight on high-speed equipment and not by count, it is possible to occasionally have an unusual color distribution. Also, because of the difference in the sizes of the individual candies, the number of pieces per package varies. However, the package should be the indicated weight. Based on knowing the percentage of each color that should be in plain M&M bag, calculate how many of each color your group should have. In other words, if between you and your lab partner you had a total of 67M&M’s, 20% of them should be brown. In order to calculate how many brown M&M’s you should have, take 20.0% of 67 (0.20 x 67 = 13.4 13 brown M&M’s). You will put all of your answers in correct significant figures (your percentages tell you how many sig figs to use). Complete the chart by calculating how many of each colored M&M’s you should have. These are your ACCEPTED VALUES. This X This Red 20.% Orange 15.% Yellow 20.% Green 15.% Blue 10.% Brown 20.% TOTAL NUMBER OF M&M’s for your lab group Now, calculate the percent error for each color of M&M. Remember: the experimental data is how many M&M’s of each color you have sitting in front of you, while the accepted value is the number of M&M’s that the M&M/Mars candy company says should be in the sample. Show your work for each color in the data table. Red Orange Yellow Green Blue Brown Work for calculating % error goes here Answer for % error goes here You will now be calculating the average atomic mass for each isotope (color) of M&M’s. Just like the periodic table you cannot simply add up all the M&M isotopes and divide by the number you added. Instead we need to find the % abundance (how often you can find each isotope in the world) for each M&M isotope. You will then mass each M&M isotope. The next step would be to change the % to a decimal and multiply that decimal by the mass. After you add up all the masses and check for significant figures you will have the average atomic mass for your M&M element. Red Orange Yellow Green Blue Brown Red Orange Yellow Green Blue Brown Using your total number of M&M’s from your lab group find the % of each color here Using your M&M’s from your lab group find the mass of each color here Red Answer Orange Yellow Green Blue Brown Average Atomic Mass for M&M’s goes here Change % to a decimal then multiply by mass goes here Analysis Questions: You must show all work in order to receive full credit! 1. What is percent error and why is it important to calculate? 2. A 1-lb. bag of M&M’s contains 600 pieces of candy. According to the M&M Company, how many green M&M’s would you expect in the bag? 3. A bag of Skittles contains 58 pieces of candy. Thirteen are purple. If the Skittles candy company states that 20% of all their candies are supposed to be purple, calculate the % error in this bag of candy. 4. Calculate the average atomic mass for silicon if 92.21% of its atoms have a mass of 27.98 amu, 4.7% have a mass of 28.98 amu, and 3.09% have a mass of 29.97 amu.