Lab1

Justin Jansen
Chemistry 112
Hard Water Analysis
02/01/2013
Purpose:
The purpose of this experiment is to determine the hardness of a water sample.
Procedure:
1.
In four separate Erlenmeyer flasks add 25.0 mL of Ca 2+ solution.
2.
In four separate Erlenmeyer flasks add 25.0 mL of tap water.
3.
Add 1 mL of buffer (pH =10) solution into each of the flasks.
4.
Add 2 drops of EBT indicator into each of the flasks.
5.
Swirl flask till the liquid inside turns purple.
6.
Clean and fill 50 mL buret EDTA solution. Record initial reading.
7.
Use the first of the four flasks of Ca 2+ solution as a practice run and titrate till the solution turns sky blue.
8.
Titrate the remaining three flasks of Ca 2+ solution. For each record both the starting and ending volume of the buret.
9.
Repeat step 7 with a flask of tap water.
10.
Repeat step 8 with the remaining three flasks of tap water.
Pre-Laboratory Questions:
1.
What cations are responsible for water hardness?
The cations responsible for water hardness are Ca 2+ and Mg 2+ .
2.
Experimental Procedure, Part A.1. Calculate the mass of disodium ethylenediaminetetraacetate
(molar mass = 372.24 g/mol) required to prepare 250 mL of 0.010 M solution. Show the calculation. Express the mass to the correct number of significant figures. m
(
g
)÷
M.W.
(
g
)
=
M
→
M
×
L
×
M.W
(
g
) =
m
(
g
)
L
0.010
×
0.250
×
372.24
=
0.9306
≈
0.93
g of Na
2
H
2
Y
3.
Experimental Procedure, Part A.3. A 25.7-mL volume of prepared Na
2
H
2
Y solution titrates 25.0 mL of standard 0.0107 M Ca 2+ solution to Eriochrome Black T endpoint. What is the molar concentration of the Na
2
H
2
Y solution? mol Ca
L Ca
= M Ca
→
M of Ca ( L of Ca )= mol Ca = mol Na
2 mol of Na
L of Na
2
2
H
H
2
2
Y
Y
= M of Na
2
H
2
H
2
Y
Y
→
M of Ca ( L of Ca )
L of Na
2
H
2
Y
= M of Na
2
0.0107
( 0.025
)
= 0.0104
M Na
0.0257
2
H
2
Y
H
2
Y
4.
a.
Which hardening ion Ca 2+ or Mg 2+ , binds more tightly to (forms a stronger complex ion with) the Eriochrome Black T indicator used for today's analysis?
Periodic trends show that as you move down the rows the lattice energy decrease.
Therefore, Mg should have a higher lattice energy then Ca and because of this Mg will form a stronger complex ion with the Black T indicator. b.
What is the color change at the endpoint?
Sky Blue.
5.
A 50.0- mL water sample requires 16.33 mL of 0.0109 M Na
2
H
2
Y to reach the Eriochome Black T endpoint. a.
Calculate the moles of hardening ions in the water sample.
1 mol Na
2
H
2
Y = 1 mol CaCO mol / L = M
→
M × L = mol
3
0.0109
× 0.01633
= 1.78e-4 mol of Na
2
H
2
Y = 1.78e-4 mol CaCO
3 b.
Assuming the hardness is due exclusively to CaCO
3
, express the hardness in mg CaCO
3
/L sample. mol of CaCO
3
× M.W of CaCO
3
= m ( g ) of CaCO
3 m ( g )× 1000 = m ( mg )
1.78e-4 × 100.088
× 1000
50mL / 1000
= 356.3
mg CaCO
3
/ L c.
What is the hardness concentration expressed in ppm CaCO
3
?
1 mg CaCO
3
/L = 1 ppm
356.3 ppm of CaCO
3
6.
d.
Classify the hardness of this water according to Table 9.1.
Very hard water a.
Determine the number of moles of hardening ions present in a 100-mL volume sample that has a hardness of 58 ppm CaCO
3
.
58 ppm =
58 mg
×
1 L
1 g
1000 mg
×
1 mol
100.088
g
5.79e-4 mol / L × 0.1
L = 5.79e-5 mol
= 5.79e-4 mol / L = 5.79e-4 M b.
What volume of the 0.100 M Na
2
H
2
Y is need to reach the Eriochorme Black T endpoint for the analysis of the solution.
Ratio 1 : 1 ( 1 mol CaC0
3
) : ( 1 mol Na
M = mol / L
→
L = mol / M
2
H
2
Y )
L of Na
2
H
2
Y =
5.79e-5
= .58
mL
0.100
Data Section: c.
Water hardness is also commonly expressed in units of grains/gallons, where 1 grain/gallon equals 17.1 ppm CaCO
3
. Express the hardness of this "slightly hard" water sample in grains/gallon.
58 ppm/ 17.1 ppm = 3.39 gpg
A. A Standard EDTA solution
B. Analysis of Water Sample
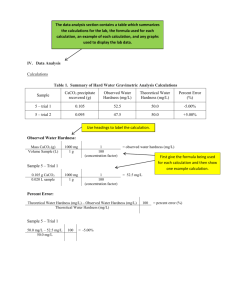
Conclusion:
In order to determine the hardness of the tap water within the chemistry lab, two primary steps where required. First, a known concentration of approximately 0.002 M CaC0
3
solution was used in order to find the concentration of an EDTA indicator. That value was approximately 0.016 M. Using this information three samples of tap water where then titrated. The tap water hardness was determined to be a average of 175.3 ppm. This number indicates that the laps tap water is "Hard Water." However, it should be noted that relative standard deviation is ~8% which indicates that the results are somewhat imprecise. This could be due to inconsistencies between the four experimenters methods of performing individual tasks of the experiment.