Update - WordPress.com
advertisement

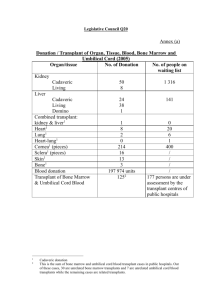
Update on Stem Cell Transplants Ottawa, June 2012 One my hospital’s hematologists gave this to our local support group. These notes have not been validated, so the usual caveats apply. After a brief history, starting with the post-Hiroshima linkage between radiation and cancers, he noted the early transplant results in the 60s were poor, largely due to lack of knowledge of blood typing and thus bad matches. In the 70s, the major development was the establishment of the international transplant registry, which collected and shared data, providing a knowledge base to inform treatment. There were relatively few transplant centres in the 1980s, but by then studies had shown that the principle of transplanting bone marrow was sound. Some of the major developments that started to improve success rates were more accurate blood typing (HLA), better antibiotics to manage complications. The previous distinction between auto SCTs, used when the underlying problem was not bone marrow related and drugs such as Neupogen made this viable, and allo, used when it is a bone marrow issue, still largely applies but, as in my case, is not iron-clad. Over the past 30 years, the main barriers to success have not changed: Original disease-related death (i.e., dying from the original cancer, about 50%) Side effects of intensive chemo and radiation, ranging from short to longer-term (about 20%) Side effects from new immune system, including GvHD and infection (risk is dramatically lower after one year) However, during this time, improvements are significant: Accurate blood typing given improved technology: if match, it really is a match Better handling of GvHD given huge advances in drugs (e.g., immunosuppressants) Development of peripheral blood cell collection, which provides for a quicker recovery than bone marrow cells (quicker recovery means lower risk of infections) and a more active system. The price to pay (there always is a trade-off is greater risk of GvHD). In general, for more aggressive cancers, the quicker recovery and increased new immunity is preferred; for less aggressive cancers, bone marrow cells may be used to reduce the risk of GvHD. Neupogen, the drug used to stimulate white blood cell production and reduce the duration of low immunity and vulnerability New anti-fungal drugs to reduce risks without ‘wrecking’ kidneys And a number of simple things: Zofran for nausea, CMV (Cytomegalovirus) monitoring to prevent pneumonia, now replaced by PCR (Polymerase chain reaction) testing with a shorter time required for Acyclovir (anti-viral drug) Some other changes flagged: Pendulum keeps shifting between harsh and less harsh conditioning regimes, and is currently swinging back to the full myeloblative regime that I had. High radiation, in particular, keeps one’s old immune system at bay for about 6 months, enough time for the new system to strengthen. In general, the bias is to do transplants sooner rather than later, as the timing affects the success rate. However, complementary treatments and drugs can postpone the need for transplant Former hard rules about age limits for transplants have shifted to looking at individual patient health (physiological age, not chronological – is the patient strong enough?), combined with an aging population, and higher treatment expectations Greater understanding of the spectrum and variants of cancers, and increased complexity of treatment Overall risk of death from treatment has decreased from 40 to 20 percent from 1995-2004, due to better patient selection, better drugs and better supporting care and managing complications. Overall survival rates have increased 12 to 15 percent over this period, incremental but in the right direction. And some additional ongoing challenges, in addition to ongoing improvements: Providing care for ‘survivors’ as longer-term effects of treatment and transplant emerge Improved cost and other efficiencies in providing care Analysis of clinical trials to adjust treatment and transplant approaches