HELA Cell Line Risk Assessment
advertisement

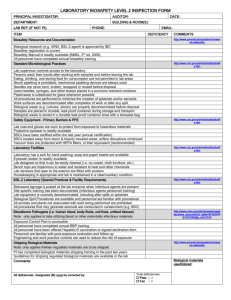
Overall Risk Group (1) Primary Hazards (2) Special Hazard Notes (2) Containment Requirements (2) Required PPE (2) Spills outside BSC (2) Effective Decontamination (2) Post Exposure Information Medical Surveillance WSU Oversight HELA Cell Line (HPV-18) Human Papilloma Virus-18 2 Exposure of mucous membranes to droplets/aerosols Accidental parenteral inoculation None Biosafety level 2 practices and containment facilities for all activities involving cell lines known to contain this virus. Laboratory coat; gloves Allow aerosols to settle: wearing protective clothing gently cover the spill with absorbent paper towel and apply sodium hypochlorite to the spill, starting at the perimeter and working towards the center; allow sufficient contact time (30 min) before clean up Sensitive to 1% sodium hypochlorite and 2% glutaraldehyde There is no prophylactic treatment once an exposure has occurred. (2) HPV-18 virus has been associated with the following diseases: Squamous cel carcinoma of the sinuses, oral carcinoma, oral leukoplakia, squamous cell carcinoma of the esophagus, Bowenoid papulosis, Bowen disease, Carcinoma of the vulva, Carcinoma of vagina, Carcinoma of cervix, Carcinoma of the penis. (4) HPV type 18 positive cervical cancer patients, despite negative histological findings in the lymph nodes shoud be considerees as a subpopulation for poor outcome especially in the young age group (5) A vaccine is currently available. This vaccine is not effective post exposure. All who work in labs that manipulate this agent are encouraged to become informed as to the appropriateness of this vaccine for them. See references for more information under (3) below. 1. Submit a BAF (Biosafety Approval Form) to the IBC (Institutional Biosafety Committee). This form can be downloaded at the following website: http://www.bio-safety.wsu.edu/forms.asp . 2. Obtain approval from the IBC before starting work. 3. Submit a current BSL-2 Biosafety Manual that includes an Exposure Control Plan for Established Cell Lines to the WSU Biosafety Manager by the submission deadline date. The BSL-2 manual template can be downloaded from the following website: http://www.biosafety.wsu.edu/forms.asp . 4. You will be contacted by EH&S to schedule a facility review. Note: The Biosafety Manual and the facility review need to be completed prior to the IBC meeting or the BAF will likely be deferred until these reviews have been satisfactorily completed. 1. HELA cell line WSU RG Risk Assessment located in this Biosafety Manual 2. http://www.phac-aspc.gc.ca/msds-ftss/msds85e-eng.php 3. http://caonline.amcancersoc.org/cgi/content/full/57/1/7 http://www.cdc.gov/std/Hpv/STDFact-HPV-vaccine.htm Page | 1 http://www.gardasil.com/ http://www.cdc.gov/std/Hpv/STDFact-HPV-vaccine.htm http://www.cancer.gov/cancertopics/factsheet/prevention/hbp-vaccine 4. http://www.emedicine.com/MED/topic1037.htm 5. http://cat.inist.fr/?aModele=afficheN&epsidt=1344446 Page | 2