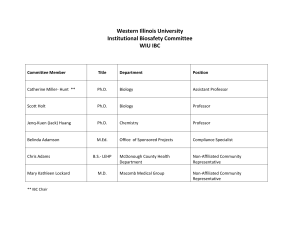
Recombinant DNA Registration Form
advertisement

BIOSAFETY OFFICER USE ONLY Registration #: Initiation Date: IBC Approval Date: Expiration Date: UNH Institutional Biosafety Committee Recombinant DNA Registration Form This form is for registration of recombinant DNA experiments and work with transgenic animals and plants. A registration for infectious work that is not recombinant is submitted on the Infectious Agents Registration Form found on the OEHS Biosafety website. For any questions about this form, or for help completing the form, please contact the Biological Safety Officer at 862-0197. Section 1: Principal Investigator Information Name: Department: Office (Building and room #): Laboratory Contact Person: Source of Funding: Phone: Position: Phone: Requested Start Date: End Date: Note: the IBC approves projects for up to a maximum of 3 years. After 3 years, a new registration will need to be filed. Section 2: Project Description Project Title: Project Description: (provide a brief summary of project goals stated in nontechnical terminology) Experimental Design: (provide a technical description of experiments; information provided must be sufficient to eliminate the need to reference to other documents or scientific papers) Requested Biosafety Level (BSL): ☐BSL-1 ☐BSL-2 ☐BSL-3 Choose an item. NIH Guidelines Section: If choosing Section III-E or III-F, a subsection must be determined and listed here: Note: Details about each section can be found in the NIH guidelines at http://oba.od.nih.gov/oba/rac/Guidelines/NIH_Guidelines.htm. 7/18/2013 Section 3: Recombinant Material Specifics Source of the DNA/RNA (name of gene and source organism): If viral, does the insert represent >2/3 of the viral genome? What is the biological activity of the gene product or sequence inserted? Does it code for a toxic product? ☐Yes ☐Yes ☐No ☐No Will a drug resistant trait be transferred to an organism that does not acquire it normally? ☐Yes ☐No Will there be an attempt to express a foreign gene? If yes, what protein is produced? ☐Yes - Protein produced: ☐No Host organism(s) for propagation (Genus, species, parent strain, cell line): ☐Prokaryotic: ☐Eukaryotic: Vector(s) used: If a viral vector is used, please complete the following: Virus Type (retrovirus, adenovirus, etc.): Virus Name: Explain the packaging system. Include whether or not a helper virus is used: Is the tropism altered? How (narrowed/expanded)? Is the viral vector defective or replication incompetent? Is testing for replication competent virus (RCV) done? Will the rDNA protocol ☐Yes - Explanation: involve animals, whole ☐No plants (including transgenics) or humans? Explain and list approval numbers as necessary. Scale of work (bench scale is <9.9 liters): 7/18/2013 Section 4: Laboratory Laboratory Building and Room Numbers (where work will be performed) Current Biosafety Level of Room (e.g. None, BSL-1, BSL-2, BSL-3) Personal Protective Equipment (currently in use) Section 5: Personnel List all personnel working on the project and briefly note their experience and/or proficiency in working with the proposed agents. Name Experience/Proficiency Safety Training Completed (date/type) Section 6: Work Practices Disinfection Surface Disinfectant Used: Liquid Waste Disinfection: Solid Waste Disinfection: Hazardous Procedures List all procedures that may cause splash, aerosol or sharps hazards (e.g. sonication, centrifugation, vortexing, needles, razors, scalpels): Control equipment available to reduce aerosol, splash or sharps injury exposure risk (BSC, centrifuge cups, bench shield): Will there be a deliberate or possible release of recombinant materials to the environment? Section 7: Materials Transport Will biological materials be transferred from one lab to another? Will materials be shipped offsite or received from a collaborator? Has a Materials Transfer Agreement been approved? Do you know if permits are required (APHIS, CDC, USDA Select Agents)? If yes, which permit is required? 7/18/2013 ☐Yes ☐Yes ☐Yes ☐Yes ☐No ☐No ☐No ☐No ☐ Not Sure ☐Yes ☐No Section 8: Risk Assessment A risk assessment must be performed by the Principal Investigator and Biological Safety Officer prior to submittal to the IBC. Contact OEHS, 862-4041, ehs@unh.edu to schedule. Section 9: Additional Information Please add any additional information relevant to this rDNA registration in this section. Examples are: additional personnel, training information, pathogens that are relevant to the work but are not recombinant, etc. 7/18/2013 Section 10: Principal Investigator’s Statement As Principal Investigator I certify that this application is accurate and complete. I understand that a risk assessment must be performed by me in conjunction with the Biological Safety Officer prior to review by the Institutional Biosafety Committee (IBC). I agree to comply with all requirements pertaining to the use, handling, storage, and disposal of recombinant and biohazardous materials. I also agree to follow the recommendations from the current edition of the CDC/NIH handbook, Biosafety in Microbiological and Biomedical Laboratories. I agree to update this Project Registration Document whenever personnel or materials change, or annually at a minimum. I understand that the IBC grants approval for up to 3 years and a new application will need to be submitted following expiration of this protocol. I also understand that I am responsible for the safe, responsible conduct of my program and design and implications of the projects under my direction. Appropriate engineering controls and personal protective equipment will be provided to all laboratory workers as necessary for the procedures required in the experiment. Any vaccinations or medical surveillance requirements, as determined by the IBC, will also be met prior to the initiation of experimental work. I will comply with shipping and permitting requirements for infectious agents and biohazardous materials. I understand that I must report any personnel exposure or biohazardous material release to the Biological Safety Officer immediately. I acknowledge that IBC registration and approval represents only institutional approval of the protocol as it relates to biosafety issues. Approval is not transferrable to any other UNH faculty or staff member. I understand that I am responsible to assure that work under this protocol complies fully with the NIH Guidelines for Research Involving Recombinant DNA Molecules and all applicable UNH policies and procedures. All other necessary institutional approvals are not covered by this registration. X Signature [To electronically sign document, double click “X” and type in your name] 7/18/2013