HW6 - University of St. Thomas
advertisement

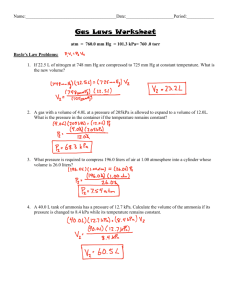
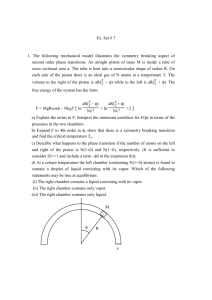
Physics 410 Spring 2014 HW#6 Due Wednesday, 19 February 2014 1. 2. 3. 2 moles of ideal argon gas occupies a volume of 50 liters at a temperature of 280K. It is expands reversibly against a piston to a volume of 100 liters. Determine the work done by the gas if this is done: a) at constant pressure; b) at constant temperature; c) in such a way that there is no heat added to the gas (adiabatically). Carter, 3-3 plus my part d): An ideal gas originally at a temperature T1 and pressure P1 is compressed reversibly against a piston to a volume equal to one-half its original volume. The temperature of the gas is varied during the compression so that at each instant the relation P = AV is satisfied where A = constant. a) Draw a diagram of the process in the P-V plane. b) Find the final temperature T2 in terms of T1. c) Find the work done on the gas in terms of n, R, and T1. d) If the gas is monoatomic, determine the heat removed from the gas during this process in terms of n, R, and ΔT. Show that the relationship between Pf, Pi, Vi, and Vf in an adiabatic expansion of an ideal gas is 1+ 2 𝑑𝑓 𝑃𝑓 𝑉𝑖 =( ) 𝑃𝑖 𝑉𝑓 [Note that what the book calls υ is actually (volume)/(number of kilomoles).] Physics 410 Spring 2014 HW#6 Due Wednesday, 19 February 2014 1. 2. 3. 2 moles of ideal argon gas occupies a volume of 50 liters at a temperature of 280K. It is expands reversibly against a piston to a volume of 100 liters. Determine the work done by the gas if this is done: a) at constant pressure; b) at constant temperature; c) in such a way that there is no heat added to the gas (adiabatically). Carter, 3-3 plus my part d): An ideal gas originally at a temperature T1 and pressure P1 is compressed reversibly against a piston to a volume equal to one-half its original volume. The temperature of the gas is varied during the compression so that at each instant the relation P = AV is satisfied where A = constant. a) Draw a diagram of the process in the P-V plane. b) Find the final temperature T2 in terms of T1. c) Find the work done on the gas in terms of n, R, and T1. d) If the gas is monoatomic, determine the heat removed from the gas during this process in terms of n, R, and ΔT. Show that the relationship between Pf, Pi, Vi, and Vf in an adiabatic expansion of an ideal gas is 1+ 2 𝑑𝑓 𝑃𝑓 𝑉𝑖 =( ) 𝑃𝑖 𝑉𝑓 [Note that what the book calls υ is actually (volume)/(number of kilomoles).]